39 thermochemistry problems worksheet number one answers
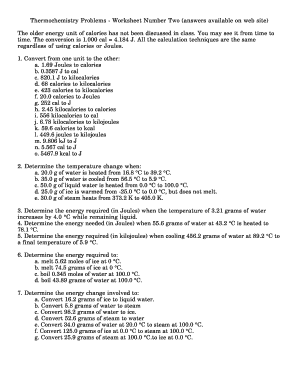
Thermochemistry Calculations Worksheet 1 - Math Writing ... 1 Cgraphite O2g H CO2g. 2S 3O 2 2SO 3 H -7914 kJ 2. Thermochemistry problems worksheet number one. Thermochemistry review part 1 definition questions. Thermochemistry Worksheet 1 Answers. Calculate the energy associated with this reaction. What is the heat change when 472 g of Carbon reacts with excess O 2 according to the following equation. Problems I - Thermochemistry Problems - Worksheet Number ... Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0°C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released?3.
Thermochem WS #1 Answers - ChemTeam Thermochem WS #1 Answers Thermochemistry Answers - Worksheet Number One We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 °C) (2.02 J/g °C).

Thermochemistry problems worksheet number one answers
Thermochemistry Problems Displaying top 8 worksheets found for - Thermochemistry With Answers Current Collection of Virtual Lab Problems Visual Metronome Handout #1: Tips on how to solve problems in thermo-fluids engineering 02 kJ/mol Molar heat of vaporization for water is 40 Thermochemistry Guided Practice Problems This is likewise one of the factors by obtaining the ... PDF Answers, Thermochemistry Practice Problems 2 Answers, Thermochemistry Practice Problems 2 1 6. When 26.7 g of H 2 S was burned in excess oxygen, 406 kJ was released. What is H for the following equation? 2 H 2 S(g) + 3 O 2 (g) 2 SO 2 (g) + 2 H 2 O(g); H = ??? Answer: -1040 kJ Reasoning: 2 2 26.7g 0.78345 mol H S 34.08 g/mol H S, so 406 kJ was released when 0.78345 moles of H 2 S reacted. 2 2 Thermochemistry Worksheet + Answers - ChemistNate Heat and q=mcΔT (Questions 1-5) Calculating Enthalpy Change (ΔH) given heat change (Questions 6-8) Hess' Law (Questions 9, 10) Enthalpies of Formation (Questions 11-14) Bond Enthalpies (Questions 15-17) Changes of State (Questions 18-20) Potential Energy Diagrams (Question 21) Working with Unit Conversions (Question 22)
Thermochemistry problems worksheet number one answers. PDF Thermochemistry Problems Worksheet Number One Answers Thermochemistry Problems Worksheet Number One Answers Nook, or their reading apps, we can make it really easy for you: Free Kindle Books, Free Nook Books, Below are some of our favorite websites where you can download free ebooks that will work with just about any device or ebook reading app. Thermochemistry Problems Worksheet Number One The ... PDF Student Worksheet for Thermochemistry An unknown substance with an unknown mass absorbs 1500J while undergoing a temperature increase of 30°C. It's specific heat is 0.49 J/g°C. What is the mass of the substance? 3. If the temperature of 50.1 g of ethanol increases from 35°C to 87.8°C, how much heat has been absorbed by the ethanol? The specific heat of ethanol is 2.44J/g°C. 4. 17 Thermochemistry Practice Problems Answers - XpCourse (1) Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: PCl 5 (g) → PCl 3 (g) + Cl 2 (g) P 4 (s) + 6Cl 2 (g) → 4PCl 3 (g) ΔH = -2439 kJ. 4PCl 5 (g) → P 4 (s) + 10Cl 2 (g) ΔH = 3438 kJ. answer = 249 ... More › More Courses ›› View Course PDF Ch 17 Thermochemistry Practice Problem Answers PDF WS1-Thermochem Title: WS1-Thermochem Author: John Park Created Date: 1/7/2002 10:20:56 AM
PDF (Cp 0c) - srvhs.org Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3. Ground State Electron Configuration: Definition & Example 22.09.2021 · Lesson Summary. Let's review. The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels. The electrons occupying the orbitals ... Email this Story to a Friend - charlotte-engmann.de 20.05.2022 · Build an atom phet lab worksheet answer key pdf phet build motion in one dimension problem a worksheet answers america the story of us heartland worksheet pdf. Properties of Waves Virtual Lab Part 1 Go to phetcoloradoedu and click on the physics link for Wave on a String. Description this worksheet uses the intro and vector screens only. 41 build … DOC Thermochemistry Answers - Worksheet Number One Thermochemistry Answers - Worksheet Number One We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1. q = (20.0 g) (20.0 °C) (2.02 J/g °C)= 808 J.
Thermochemistry With Answers Worksheets - K12 Workbook Worksheets are Thermochemistry, Thermochemistry, Thermochemistrypractice thermochemical equations and, Thermochemistry calculations work 1, Ap chemistry review work unit 4, Answers thermochemistry practice problems 2, , Chapter 17 thermochemistry work answers. *Click on Open button to open and print to worksheet. PDF Analysis of the situation Conclusions from the above Answers, Thermochemistry Problems-1 Since the coefficient of P4 is "1" in the balanced equation, you need to find the amount of energy released when ONE MOLE of P4 is burned to get the magnitude of the H for the (thermo)chemical equation. How many moles is 3.56 g of P4?Molar mass of P4 = 4(30.97 g/mol) = 123.9 g/mol P4.So: PDF Thermochemistry Problems Worksheet Number One Answers Thermochemistry Problems Worksheet Number One Answers Author: cropover.nationnews.com-2022-04-27T00:00:00+00:01 Subject: Thermochemistry Problems Worksheet Number One Answers Keywords: thermochemistry, problems, worksheet, number, one, answers Created Date: 4/27/2022 8:19:46 AM Thermochemistry Problems Worksheet One - Key - KEY ... Thermochemistry Problems Worksheet One - Key - KEY Thermochemistry Problems \u2014 Worksheet Number One (answers available on web site 1 How much energy | Course Hero Thermochemistry Problems Worksheet One - Key - KEY... School Central Bucks High School South Course Title SCIENCE Parallel C Type Notes Uploaded By tncpatel2 Pages 4
Thermochemistry Worksheet Answer Key Thermochemistry Worksheet #1. 1. The reaction of magnesium with sulfuric acid was carried out in a calorimeter. This reaction caused the temperature of 27.0 grams of liquid water, within the calorimeter, to raise from 25.0 (C to 76.0 (C. Calculate the energy associated with this reaction. Answer: 5760J. 2. The reaction of zinc with nitric acid ...
PDF Thermochemistry Problems Worksheet Number One Answers thermochemistry problems worksheet number one answers chemistry 101science com. chemistry with lab 2018 - easy peasy all in one high school. burnout burnin sassister soi meme grace au jin shin jyutsu. download test bank for chemistry the central science 12th. printable crossword puzzles. port manteaux word maker onelook dictionary search.
PDF Thermochemistry Example Problems 1 Thermochemistry Example Problems Recognizing Endothermic & Exothermic Processes ... List the knowns & the unknown. Find the number of moles of ice that can be melted by the addition of 2.25 kJ of heat. Convert moles of ice to grams of ice. 2) Calculate - Solve for the unknown. ... Express your answer in kJ. 7 Calculating the Enthalpy Change ...
PDF Thermochemistry Worksheet 1 Answers Thermochemistry Worksheet #1 Thermochemistry Worksheet 1 Answers Thermochemistry Answers - Worksheet Number One. We will ignore any heats losses to the walls of the container and losses to the air. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. 1.
thermochemistry questions and answers - Free Textbook PDF Thermochemistry Problems - Worksheet Number One (answers available on web site). 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0. °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3. How much ... WS1-Thermochem.pdf
PDF 21. The graph below shows a pure substance which is heated ... Thermochemistry Problems - Worksheet Number One (answers available on web site) 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? 3.
PDF Chapter 17 Thermochemistry Worksheet Answers Thermochemistry With Answers Worksheets - Kiddy Math 1. How much energy must be absorbed by 20.0 g of water to increase its temperature from 283.0 °C to 303.0 °C? 2. When 15.0 g of steam drops in temperature from 275.0 °C to 250.0 °C, how much heat energy is released? Thermochemistry Problems - Worksheet Number One



0 Response to "39 thermochemistry problems worksheet number one answers"
Post a Comment