40 electron configuration level two worksheet answers
22 of the Best 10th Grade Science Projects and Experiments Check out our list of 22 science projects and experiments that you can try with your 10th graders this month. Is a Dense Fruit a Healthy Fruit? | Education.com - Grades 9-12, In this experiment, students will find out if there is a correlation between density and nutritional value, by measuring the density of vegetables and fruits. Dallas Independent School District | PowerSchool Learning | K-12 ... On Friday, Aug 5, 2022 from 7:00 PM to 11:59 PM PDT we are doing maintenance and updates to PowerSchool Learning. There may be intermittent connectivity to the aforementioned application for the duration of the maintenance window.
UNF: UNF Homepage Located on a beautiful campus just minutes from the beach, UNF offers students small class sizes and individualized attention, countless opportunities to gain real-world experience while in school, strong job placement, an active student life, Division I athletics, and a welcoming and engaged community. It's all here, and it's all Uniquely ...
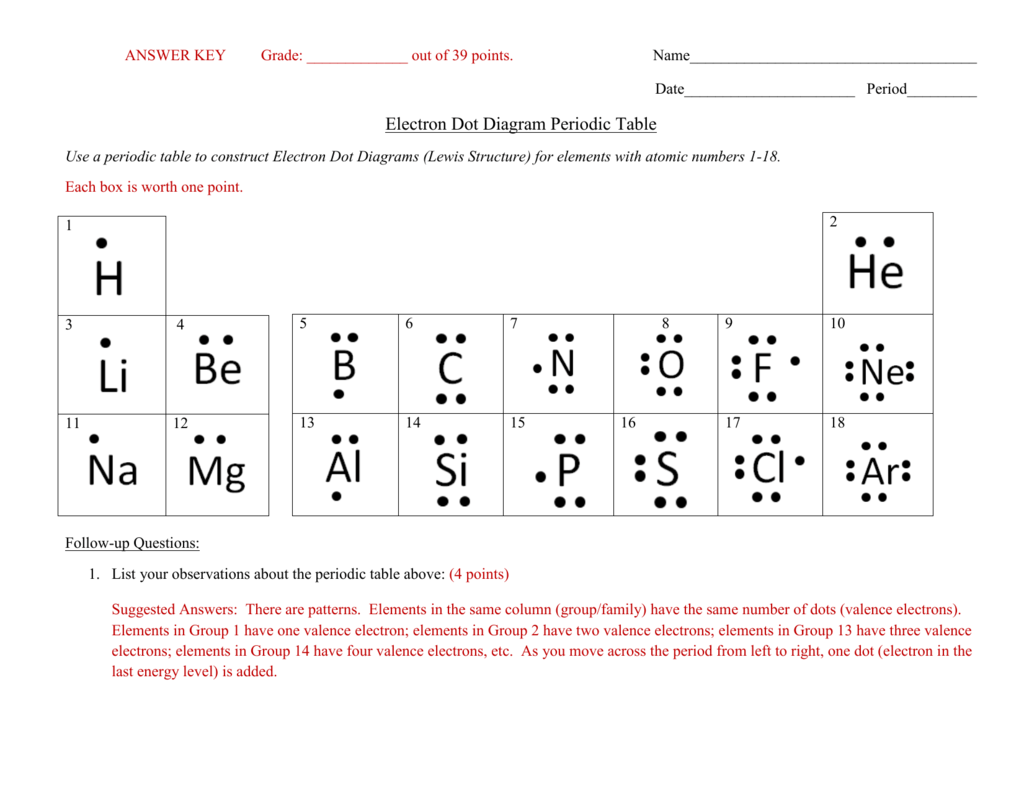
Electron configuration level two worksheet answers
9.6: Quantum-Mechanical Orbitals and Electron Configurations Write the electron configuration code for potassium. Solution This time, take a close look at Figure 9.6. 5. 1. Begin by filling up the 1 s sublevel. This gives 1s2. Now the n = 1 level is filled. 2. Next, fill the 2 s sublevel. This gives 1s22s2 3. Next, fill the 2 p sublevel. This gives 1s22s22p6. Now the n = 2 level is filled. 4. Rare-earth element - Wikipedia The actual metallic densities of these two groups overlap, with the "light" group having densities from 6.145 (lanthanum) to 7.26 (promethium) or 7.52 (samarium) g/cc, and the "heavy" group from 6.965 (ytterbium) to 9.32 (thulium), as well as including yttrium at 4.47. Europium has a density of 5.24. Origin [ edit] Crystal Field Theory - Amrita Vishwa Vidyapeetham The dx 2 -y 2 and dz 2 orbitals point along the x, y and z directions and dxy, dzx and dyz orbitals point in between x, y and z directions. The direction of approach of ligands does not coincide exactly with either the e or t 2 orbitals. The t2 orbitals are pointing close to the direction in which the e orbitals are lying in between the ligands.
Electron configuration level two worksheet answers. Maxwell's equations - Wikipedia Maxwell's equations, or Maxwell-Heaviside equations, are a set of coupled partial differential equations that, together with the Lorentz force law, form the foundation of classical electromagnetism, classical optics, and electric circuits.The equations provide a mathematical model for electric, optical, and radio technologies, such as power generation, electric motors, wireless communication ... Flowchart Tutorial ( Complete Flowchart Guide with Examples ) Answer: A computer program consists of many processes and flows. Flowcharts are used to visualize the processes and make them understandable for non-technical people. They are also used to visualize algorithms and comprehend pseudo-code which is used in programming. Comments and Feedback on the Flowchart Tutorial What is a sperm cell like? Its structure, parts and functions The head is an oval-shaped structure, which size ranges from 5 to 8 µm. It consists of two parts: Acrosome Accounts for 40% to 70% of total sperm head area, and is located at one end of the sperm cell. It contains proteolytic enzymes that help to destroy the outer layer of the egg cell, thereby allowing the sperm to enter into it easily. Nucleus Starch vs. Glycogen Function & Uses | Difference Between Starch ... Electron Configuration: Orbital, Noble-Gas & Electron-Configuration Notation ... SAT Subject Test Mathematics Level 2: Practice and Study Guide ... Quiz & Worksheet - Environmental Problems ...
Wavelength to Frequency Calculator - everything RF An important point to note is that two waves with different wavelengths can have the same frequency. So lets assume, that Wave 1 has a wavelength of 1 cm and Wave 2 has a wavelength of 2 cm. To have the same frequency, Wave 2 will need to travel at a speed that is 2 times Wave 1. Set up coordinated meetings with Microsoft Teams Rooms and Surface Hub ... Use the worksheet you created in the previous step to help you set up your devices. Use the Teams Rooms device's touch screen To set up Coordinated Meetings on a device, do the following: Select ... More > Settings. Enter the Administrator password and select Yes. Select Coordinated Meetings. Under Options, set Coordinated Meeting to on. Mr. Jones's Science Class Matter: Atoms and Properties - Open Response Question 3. Force and Motion - Open Response Question 3. Forms of Energy - Open Response Question 1. Forms of Energy - Open Response Question 2. Earth's Structure & Natural Processes - Open Response Question 1. Warehouse Order Picking and Selecting: Strategies, Types and More In addition to the five most common picking types, many companies will combine two or more to better fit the unique needs of their warehouse. For instance, managers may integrate the successful points of the above to make a zone-batch picking, zone-wave picking or zone-batch-wave picking plan that fits their changing needs best.
SBCodez - SBCodez.com - Swag Codes Posted Instantly Swagbucks UK Swag Code August 6, 2022. Posted on August 6, 2022 by Youmukon. Swag Codes: Farm25XXXX. Location: Offer Pages. Expires: Expired - 12 hours ago. Instructions: CLICK HERE TO GET YOUR CODE! ENTER IT AT SWAGBUCKS.COM. Worth: 2 SB. 8.2: Octet Rule - Chemistry LibreTexts Lithium tends to lose one electron to take on the electron configuration of the nearest noble gas, helium, leaving it with two valence electrons. There are two ways in which atoms can satisfy the octet rule. One way is by sharing their valence electrons with other atoms. The second way is by transferring valence electrons from one atom to another. Sf2 Molecular Geometry, Lewis Structure, Polarity and Bond Angles Sulfur has six valence electrons in its outer shell. Fluorine has seven valence electrons. Total number of valence electrons for SF2 - 6 + 7*2 ( as there are two atoms of Fluorine, we will multiply the number by 2) = 6 + 14 = 20 valence electrons So, Sulphur Difluoride has a total of 20 valence electrons. SF2 Lewis Structure IB Chemistry revision notes and syllabus The IBC web is a university entrance level chemistry resource with notes on standard level and higher level topics, worked example exam questions. For home study, questions, quizzes and interactive revision resources go to the Colourful Solutions Interactive Web. Happy hunting and if you like what you find don't forget to...
Mitochondrial DNA - Genome.gov Definition. …. Mitochondrial DNA is the circular chromosome found inside the cellular organelles called mitochondria. Located in the cytoplasm, mitochondria are the site of the cell's energy production and other metabolic functions. Offspring inherit mitochondria — and as a result mitochondrial DNA — from their mother.
KEAM 2022 Exam - Dates, Result, Cut Off, Counselling ... - Careers360 Commissioner Entrance Exam (CEE) Kerala conducts Kerala Engineering Architecture Medical exam every year for the admissions to Engineering, Pharmacy and Architecture courses. The KEAM exam is conducted for 2 hours and 30 minutes which consists of Multiple Choice Questions. KEAM exam 2022 paper 1 will be conducted in two shifts morning and ...
SF4 Molecular Geometry, Lewis Structure, Bond Angles and Polarity Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. There are three lone pairs on each fluorine atom. It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry. SF4 has ...
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape To get the total number of valence electrons, we will add up the valence electrons for both these atoms. Nitrogen - 5 valence electrons Hydrogen - 1 electron, but as there are 3 Hydrogen atoms we will multiply it by 3, there are three valence electrons of all Hydrogen atoms. Total number of valence electrons - 5+3 = 8 valence
HCN Lewis Structure, Molecular Geometry, Shape, and Polarity Total number of valence electrons in HCN= No. of valence electrons in Hydrogen + No. of valence electrons in Carbob+ No.of valence electrons in Nitrogen = 1+4+5 = 10 valence electrons Hence, Hydrogen Cyanide, HCN, has ten valence electrons. HCN Lewis structure
Ionization Energy - Chemistry LibreTexts 1 st, 2 nd, and 3 rd Ionization Energies. The symbol \(I_1\) stands for the first ionization energy (energy required to take away an electron from a neutral atom) and the symbol \(I_2\) stands for the second ionization energy (energy required to take away an electron from an atom with a +1 charge. Each succeeding ionization energy is larger than the preceding energy.
NCERT Solutions for Class 11 Chemistry: Download PDF - EMBIBE The solutions cover answers to all the in-text questions asked in the NCERT Chemistry textbook for Class 11. ... The worksheets and questions provided in the pdf sync well with the understanding level of any student. ... The periodic table has six groups of p-block elements, numbering from 13 to 18. Their valence shell electronic configuration ...
JetPunk - World's Best Quizzes Since 2008, JetPunk has created thousands of fun and interesting quizzes. Whether you are a trivia nut, want to expand your horizons, or just want something fun to do - we've got you covered.
Principal Quantum Number | Overview, Methods & Examples - Video ... The top row of atoms has a principal quantum number of 1. The second row of atoms has a principal quantum number of 2, and so on. The principal quantum number of an element can never be zero. The...
BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity To draw a Lewis Structure, first of all, add electrons and draw the connectivities. As discussed, here there are 24 electrons. Then, add octets to the outer atom and extra electrons to the central atom. But, as we know, there are no extra electrons. (24 - 24 = 0) Violations
Rhizaria Types & Characteristics | Forams, Radiolarians & Cercozoans ... Amoeboids, or amoebas, are organisms that are unicellular (made up of a single cell type) that are able to change their shape. Most amoebas change their shape using pseudopodia, which are thin...
Crystal Field Theory - Amrita Vishwa Vidyapeetham The dx 2 -y 2 and dz 2 orbitals point along the x, y and z directions and dxy, dzx and dyz orbitals point in between x, y and z directions. The direction of approach of ligands does not coincide exactly with either the e or t 2 orbitals. The t2 orbitals are pointing close to the direction in which the e orbitals are lying in between the ligands.
Rare-earth element - Wikipedia The actual metallic densities of these two groups overlap, with the "light" group having densities from 6.145 (lanthanum) to 7.26 (promethium) or 7.52 (samarium) g/cc, and the "heavy" group from 6.965 (ytterbium) to 9.32 (thulium), as well as including yttrium at 4.47. Europium has a density of 5.24. Origin [ edit]
9.6: Quantum-Mechanical Orbitals and Electron Configurations Write the electron configuration code for potassium. Solution This time, take a close look at Figure 9.6. 5. 1. Begin by filling up the 1 s sublevel. This gives 1s2. Now the n = 1 level is filled. 2. Next, fill the 2 s sublevel. This gives 1s22s2 3. Next, fill the 2 p sublevel. This gives 1s22s22p6. Now the n = 2 level is filled. 4.












0 Response to "40 electron configuration level two worksheet answers"
Post a Comment