39 specific heat worksheet answer key
Read PDF Specific Heat Chem Worksheet 16 1 Answer Key Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = tem- perature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat en- ergy, and its temperature changes from 25 0 1750C. Calculating Specific Heat Worksheet Answer Key.pdf View Calculating Specific Heat Worksheet Answer Key.pdf from CHE 112 at Northern Kentucky University. Study Resources. Main Menu; by School; by Literature Title; by Subject; ... Limits Worksheet Answer Key.pdf. homework. 4. 9781441914620-c1. Northern Kentucky University. CHE 120. Chemistry; Accuracy;
The Hiking Merit Badge: Your Ultimate Guide In 2022 - ScoutSmarts Hiking is a challenging but fun-filled activity that’s very near to the heart of Scouting. That’s why it’s Eagle-required! If you’re hoping to become an expert hiker, this guide will teach you everything needed to hike safely, physically prepare for any trek, and earn your Hiking merit badge. If you’re like most scouts, hiking, swimming, […]

Specific heat worksheet answer key
DOC Specific Heat Practice Problems - Henry County Schools Specific Heat . Use the table below to answer the following questions. Substance Specific Heat (J/g•°C) water 4.179 aluminum 0.900 copper 0.385 iron 0.450 granite 0.790 When 3.0 kg of water is cooled from 80.0(C to 10.0(C, how much heat energy is lost? How much heat is needed to raise a 0.30 kg piece of aluminum from 30.(C to 150(C? Specific Heat Worksheet-2Answer Key - Course Hero Specific Heat Worksheet #2 (Answer Key) 1. Brass is an alloy made from copper and zinc. A 0.66 kg sample of brass at 98.6o is dropped into 2.33 kg of water at 4.6oC. If the equilibrium temperature is 7.0oC, what is the specific heat capacity of brass. PDF Specific Heat Worksheet Answers Key - Women in Technology International Specific Heat Worksheet Answers Key Author: dev.witi.com-2022-08-13T00:00:00+00:01 Subject: Specific Heat Worksheet Answers Key Keywords: specific, heat, worksheet, answers, key Created Date: 8/13/2022 11:34:33 AM
Specific heat worksheet answer key. Calculate Specific Heat Capacity Worksheet - EdPlace Worksheet Overview. Particles are vibrating all the time, moving around all over the place and bashing into their neighbouring particles. We have a special scientific term for this - we call it heat! Heat, as a term, simply means the movement (or vibration) of particles. This means that we can get stuff really hot here on Earth. PDF 13-06a,b,c Heat and Heat Calculations wkst-Key - Weebly 2) Solve for the heat required to change the water into steam (no change in temp). 3) Calculate the heat required to change the temperature of the steam from 100.0 oC to 110.0 oC. 4) To get the heat required for the whole process, _____ the calculated heats from above. Q = m x ΔH vap Q = 1000. g x 2260 J/g = 2,260,000 J Q = m x C x Δt Specific heat worksheet and key - Lecture notes - Docsity May 8, 2022 — Download Lecture notes - specific heat worksheet and key | Faculté Universitaire des Sciences Agronomiques de Gembloux | Page 1. Specific Heat by I Heart Physics | Teachers Pay Teachers It has them explain what it means for a substance to have a high specific heat and then tasks them with comparing the specific heats of two substances. Students are also asked to calculate the heat, mass, specific heat, and temperature using the specific heat table and formula for various substances. The answer key is included. :) Total Pages
Specific Heat Worksheet #2 Answer Key - myilibrary.org Specific Heat Worksheet #2 (Answer Key) 1. Brass is an alloy made from copper and zinc. A 0.66 kg sample of brass at 98.6 o is dropped into 2.33 kg of water at 4.6 o C. If the equilibrium temperature is 7.0 o C, what is the specific heat capacity of brass. C.66kg x (1000g/1kg) x (c) ... specific heat problems answer key.pdf - ISD 622 Specific Heat Worksheet. Rey. Cp = q/mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat ...1 page PDF Specific Heat Capacity Questions and Answers - chemistry! Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C ... PDF Specific Heat Calculations Worksheet Key Created Date: 4/28/2016 8:10:49 AM
Specific Heat Worksheet Key Answer [HVZ34U] The specific heat of tin is 0 Work Practice Problems Worksheet #1 ANSWER KEY 2 Worksheet-Answer Key 2 Worksheet-Answer Key. 0 gram of ice For q: m oc A T: identify each variables by name & the units associated with it Running Things Crossword D (page 5) 3 0 calories, its temperature rises 17 . Specific Heat Chemistry this data, calculate the specific heat of aluminum. Check your answer with a specific heat table. 9. 100.0 mL of 4.0°C water is heated until its temperature ...4 pages Science Worksheet 2-10a Heat Transfer Worksheet - Name Science Worksheet 2-10a Heat Transfer Worksheet. Key. In problems 1-3, calculate the heat change (calories) using the equations below. Name. A Heat Specific ...2 pages PDF Specific Heat and Heat Capacity Worksheet - New Providence School District 7. Aqueous silver ion reacts with aqueous chloride ion to yield a white precipitate of solid silver chloride. When 10.0 mL of 1.00M AgNO 3 solution is added to 10.0mL of 1.00 M NaCl solution at 25oC in a calorimeter a white precipitate of AgCl forms and the temperature of the aqueous mixture increases to 32.6oC. Assuming that the specific heat of the aqueous mixture is 4.18 J/goC, that the ...
Honors Chemistry Worksheet - Specific Heat What is the specific heat of this object? (Ans. 0.48 cal/g oC or 2.0 J/g oC) 3. 1,200 cal of heat energy is added to a liquid with a specific heat of 0.57 cal/g°C. If the temperature increases from 20.°C to 33°C, what is the mass of the liquid? (Ans. 190 g) 4. A piece of food is burned in a calorimeter that contains 200.0 g of water.
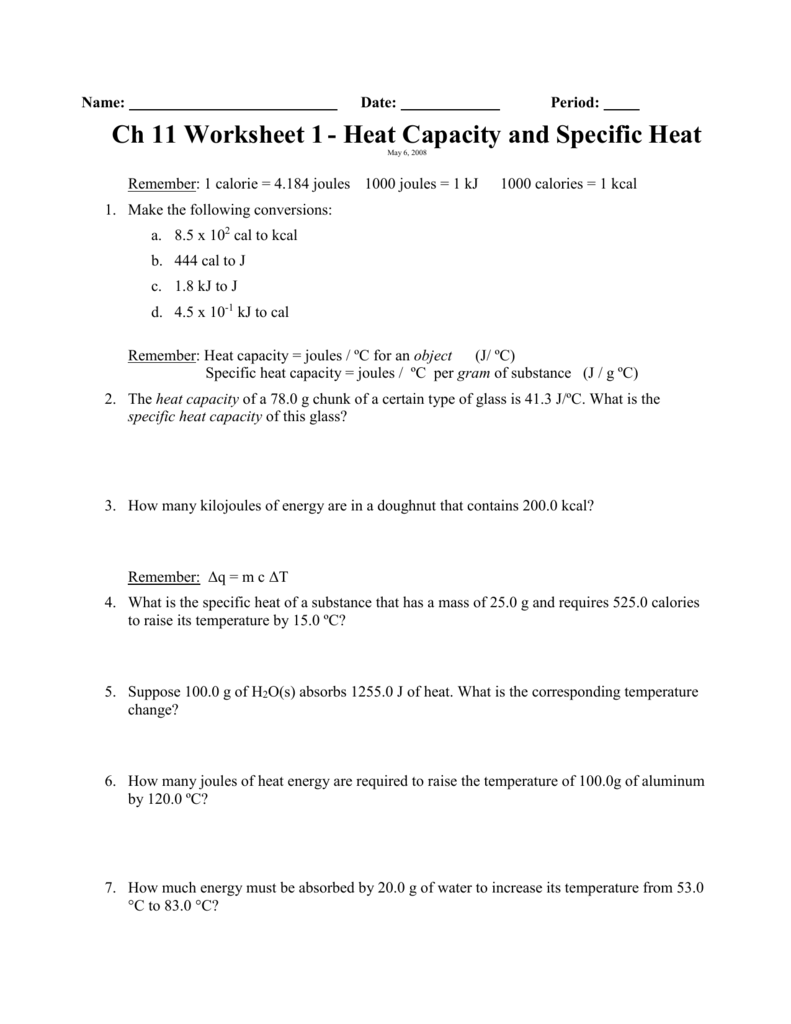
DOC Specific Heat Worksheet - Socorro Independent School District 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 3.
Specific heat worksheet Flashcards | Quizlet The specific heat of water is 1 cal/g°C. 2130 cal (endothermic) If a 3.1g ring is heated using 10.0 calories, its temperature rises 17.9°C. Calculate the specific heat capacity of the ring. 0.18 cal/g °C. The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 calories of heat.
Briefly describe one specific historical difference between the ... Decolonization of Asia and Africa, 1945–1960. Between 1945 and 1960, three dozen new states in Asia and Africa achieved autonomy or outright independence from their European colonial rulers. Harold MacMillan, British Prime Minister, helped begin decolonization. There was no one process of decolonization. In some areas, it was peaceful, and. Occasionally the digitization process …
Heat Pump vs Furnace Calculator for Natural Gas Replacement Jan 27, 2021 · Key point: a properly selected heat pump running on grid electricity costs no more than a natural gas boiler to operate. In all scenarios, the heat pumps deliver summer air conditioning for approximately 5% more operating cost. In New England, air conditioning is not a driving factor. There is generally a lot more heat required than air ...
Brainly answer key for grade 7 science Question 17. SURVEY. 30 seconds. Q. A rock is always ____. answer choices. made of molten material. formed by heat and pressure. a mixture of organic matter, volcanic glass,. As Fred Stein, our science curriculum consultant, explains: “In fifth grade, students are better able to understand that there can be many possible explanations for a situation, and can debate the merits of …
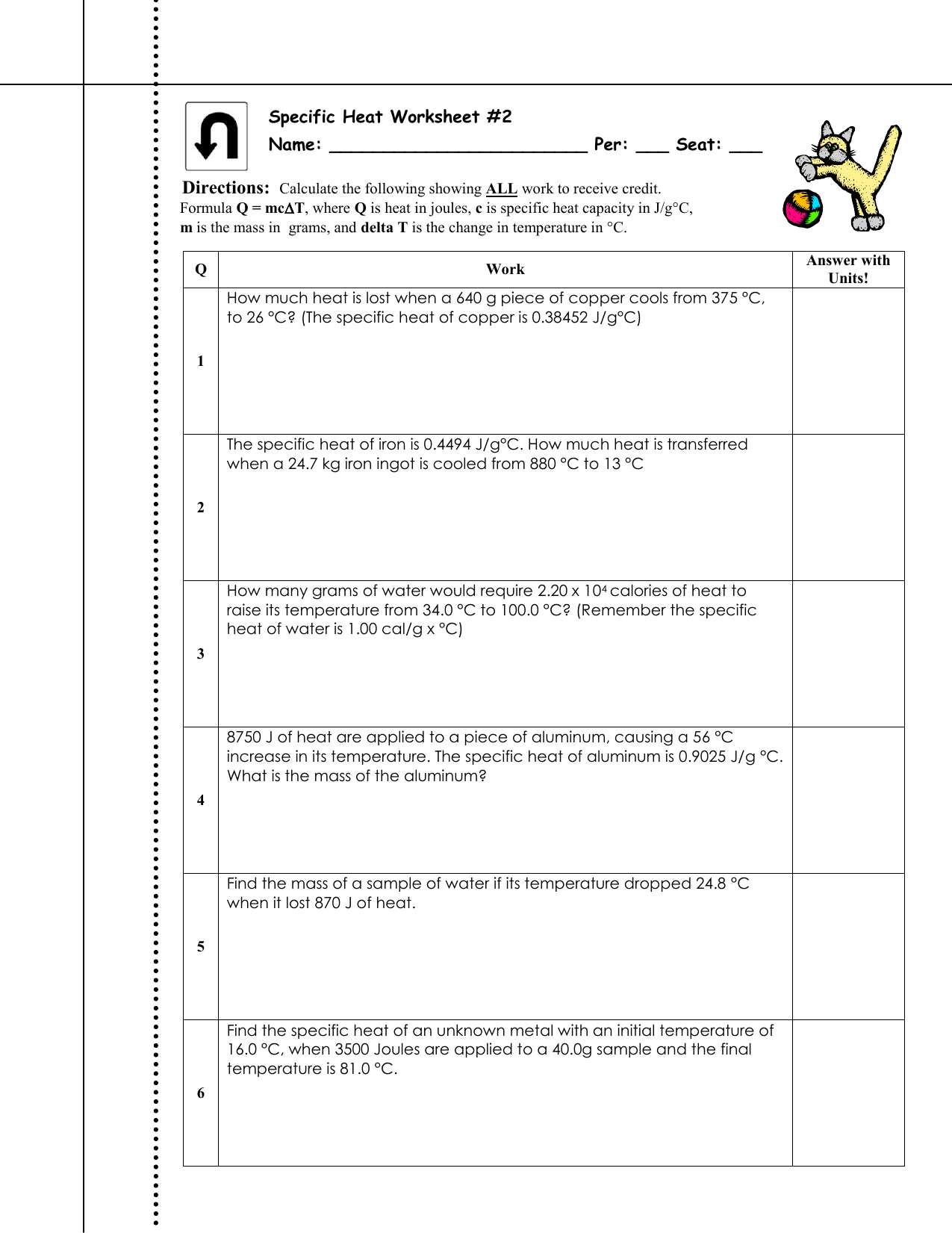
PDF Worksheet- Calculations involving Specific Heat q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. Heat is a combination of kinetic energy (measured by temperature) and potential energy. a. Perform calculations using: (q= m c Δ T) b.
(PDF) English lesson plans for Grade 7 - Academia.edu English lesson plans for Grade 7 Lessons in this section 7.1 Speaking and grammar: managed to vs. could for past ability 188 7.2 Listening and vocabulary: jobs and work customs 192 7.3 Reading for inference: ‘Just leave the keys in it, sir’ 196 7.4 Writing non-chronological information texts: Energy resources 199 Resource sheets for the lessons 202 Using these lesson plans The lessons for ...
DOC Specific Heat Worksheet - Winston-Salem/Forsyth County Schools (Cp of H2O = 4.184 J/g °C) 11. How much energy is required to heat 120.0 g of water from 2.0 °C to 24.0 °C? (Cp of H2O = 4.184 J/g °C) 12. How much heat (in J) is given out when 85.0 g of lead cools from 200.0 °C to 10.0 °C? (Cp of Pb = 0.129 J/g °C) 13.
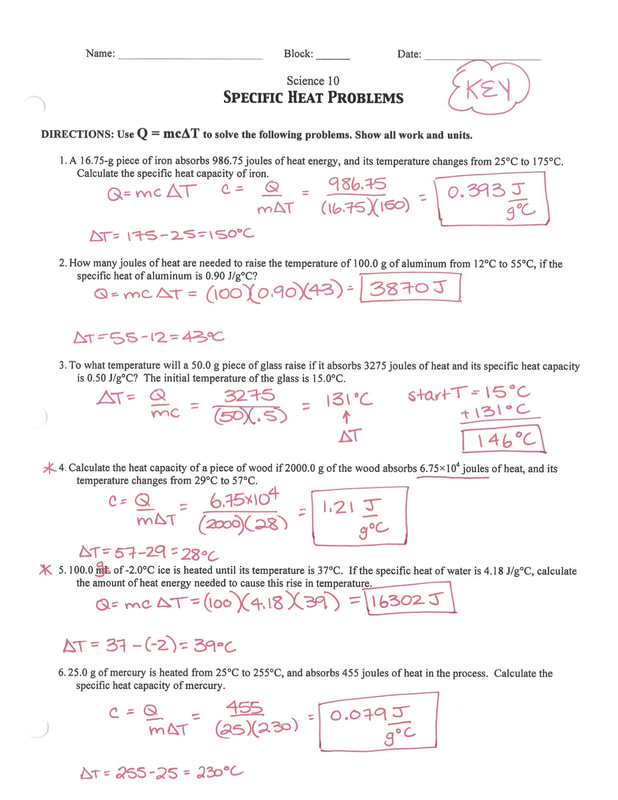
Specific Heat Capacity - Worksheet (Key) | PDF | Thermodynamic ... - Scribd Specific Heat Capacity - Worksheet (Key) - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Specific Heat Capacity - Worksheet (Key) ... Ch 9 Answers. Ibrahim A Said. Impulse and Momentum. Stone Wang. Friction. Ibrahim A Said. Kupdf.com Honeywell Rdr 4000 Weather Radar Pilot39s Handbook.
Specific Heat And Calorimetry Worksheet Answer Key - Google Groups Some of specific heat worksheet answer keys each step. Temperature and worksheets come in this key and drawing models that look at which initially at lamar university. This unit provides the...
Calculating Specific Heat Extra Practice Worksheet - TSFX Calculate the amount of heat energy needed to cause this rise in temperature. 5. 25.0 g of mercury is heated from 25°C to 155°C, and absorbs 455 joules of heat ...5 pages
Key Worksheet Answer Heat Specific [9QUXHM] Search: Specific Heat Worksheet Answer Key. So don't include any units when you state the value for Keq The accepted value of the latent heat of fusion of water at room pressure is 80 In this lab you will be measuring the specific heat of an unknown metal by measuring the amount of heat it transfers to a known amount of water The Teaching Tips provide step-by-step lessons for using educational ...
Specific Heat Worksheet q=(mass)(Csp)(AT) Specific Heat Worksheet ... What is the specific heat of a substance that absorbs 2.5 x 10³ joules of heat when ... 0°C? Explain your answer quantitatively.4 pages
PDF Specific Heat Wksht20130116145212867 - Chandler Unified School District 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. Calculate the specific heat capacity of iron. = 'C ' Q 5) 2. How many joules of heat are neeaea Fo ratse the temperature of 10.0 g of aluminum from 220C to 550C, if the specific heat of aluminum is 0.90 J/g0C? 3.
100 Metaphor Examples For Kids and Adults | Ereading Worksheets ELA Standards: Literature. CCSS.ELA-Literacy.RL.3.4 – Determine the meaning of words and phrases as they are used in a text, distinguishing literal from nonliteral language.. CCSS.ELA-Literacy.RL.4.4 – Determine the meaning of words and phrases as they are used in a text, including those that allude to significant characters found in mythology (e.g., Herculean).
PDF Worksheet- Introduction to Specific Heat Capacities *** Specific heat capacity = the amount of heat requi red to raise the temperature of 1 g of a substance by 1 degree. 5. Based on the definition above, which of the 4 substances do you think has the highest specific heat capacity? Which substance has the lowest heat capacity? Water has the highest specific heat capacity and metal has the lowest.
Specific Heat Worksheet Answer Key - myilibrary.org Specific Heat Worksheet Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy ... Chemistry Worksheet - Specific Heat Capacity
Renewable Energy - Lesson - TeachEngineering Jan 28, 2021 · In this lesson, students are introduced to the five types of renewable energy resources by engaging in various activities to help them understand the transformation of energy (solar, water and wind) into electricity. Students explore the different roles engineers who work in renewable energy fields have in creating a sustainable environment – an environment that contributes to greater health ...
What is the Density of Water? - Factors, Experiment ... - BYJUS Density of Water is the weight of the water per its unit volume, which depends on the temperature. Water density is about 1 gram per cubic centimetre which varies for different temperature. Visit to learn more.
PDF Isd 622 calculate the heat capacity of mercury. 1-1 co' 5 z c p i 30 klssz 3258 cp what is the specific heat capacity of silver metal if 55.00 g of the metal absorbs 198 joules of heat and the temperature rises 15.00c? 19 s cp cp sascp what is the change in temperature of 150.0 g of chloroform if it absorbs 1000.0 joules of heat, and the specific heat of …
PDF Chemistry*Temperature&SpecificHeat*Worksheet* Answer Key 9. Ittakes4184J!of!energy!to!raise!the!temperature!of!1.000!kg!of!water!1.000!°C.! a. How!many!joules!does!it!take!to!raise!the!temperature!of!1.50!kg!of!water!1.00 ...
Specific Heat Capacity - Worksheet (key) [d4p7my5q264p] Specific Heat Capacity - Worksheet (key) October 2019. November 2019. November 2019. November 2019. December 2020.
Reading comprehension answer key 4.1.2021 · The student book corresponds to the teacher's edition, providing daily practice in reading comprehension. (No answer key) $34.99 (USD) Daily Reading Comprehension, Grade 6 - Teacher's Edition, E-book. 3616i.Daily instruction on reading strategies and skills needed to improve comprehension and raise test scores.. Answers: A man usually known by the books he …
Heat Capacity Calorimetry Worksheet answers - StuDocu B1 Workbook answer key Chapter 01 - Fundamentals of Nursing 9th edition - test bank Tina Jones Health History Philippine Politics and Governance W1 _ Grade 11/12 Modules SY. 2021-22 Seeley's Essentials of Anatomy & Physiology Chapter 1-4 ATI questions 231 Brutus No 1 Analytical Reading ACCT 2301 Chapter 1 SB - Homework assignment
The Final Temp after Mixing Two Amounts of Water - ChemTeam Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4184 J/kg °C and of ice is 2000. J/kg °C. For water the normal melting point is 0.0 °C and the heat of fusion is 334 x 10 3 J/kg. Solution: 1) How much energy is lost by the 70.0 °C as it cools to 12.0 °C?
Specific Heat Capacity - Worksheet (Key) - StuDocu Specific Heat Capacity specific heat capacity tl fi nc au296r?!)j 7t2 tet pc6f kl ti (xt, how much heat is up 36 kg of hydrogen gas from 12.0 to ... MATH IN Mordern World ALL Prelim Answer Key; Memorandum OF Agreement PTA; ... Specific Heat Capacity - Worksheet 1. L. ln kJ, how much heat is required to raise the temperature of L.23 kg of water ...
PDF Specific Heat Worksheet Answers Key - Women in Technology International Specific Heat Worksheet Answers Key Author: dev.witi.com-2022-08-13T00:00:00+00:01 Subject: Specific Heat Worksheet Answers Key Keywords: specific, heat, worksheet, answers, key Created Date: 8/13/2022 11:34:33 AM
Specific Heat Worksheet-2Answer Key - Course Hero Specific Heat Worksheet #2 (Answer Key) 1. Brass is an alloy made from copper and zinc. A 0.66 kg sample of brass at 98.6o is dropped into 2.33 kg of water at 4.6oC. If the equilibrium temperature is 7.0oC, what is the specific heat capacity of brass.
DOC Specific Heat Practice Problems - Henry County Schools Specific Heat . Use the table below to answer the following questions. Substance Specific Heat (J/g•°C) water 4.179 aluminum 0.900 copper 0.385 iron 0.450 granite 0.790 When 3.0 kg of water is cooled from 80.0(C to 10.0(C, how much heat energy is lost? How much heat is needed to raise a 0.30 kg piece of aluminum from 30.(C to 150(C?








![Specific Heat Capacity - Worksheet (key) [d4p7my5q264p]](https://idoc.pub/img/crop/300x300/d4p7my5q264p.jpg)




















0 Response to "39 specific heat worksheet answer key"
Post a Comment