45 periodic trends worksheet atomic radius answers
Periodic Trends Worksheet - Houston Community College What trend in atomic radius occurs down a group on the periodic table? What causes this trend? Atomic radius decreases down the group on the periodic table. As we compare the elements down the group, the effective nuclear charge increases, but at the same time the outermost electrons are found in the shell that is farther away from the nucleus. Periodic Trends Worksheet Flashcards | Quizlet A)Atomic Radius (Excluding noble gases) B)First ionization energy C)Electronegativity A)Decrease B)Increase C)Increase What trend in atomic radius occurs down a group on the periodic table?What causes this trend? Increases down group because energy level shells are added What trend in ionization energy occurs across a period on the periodic table?
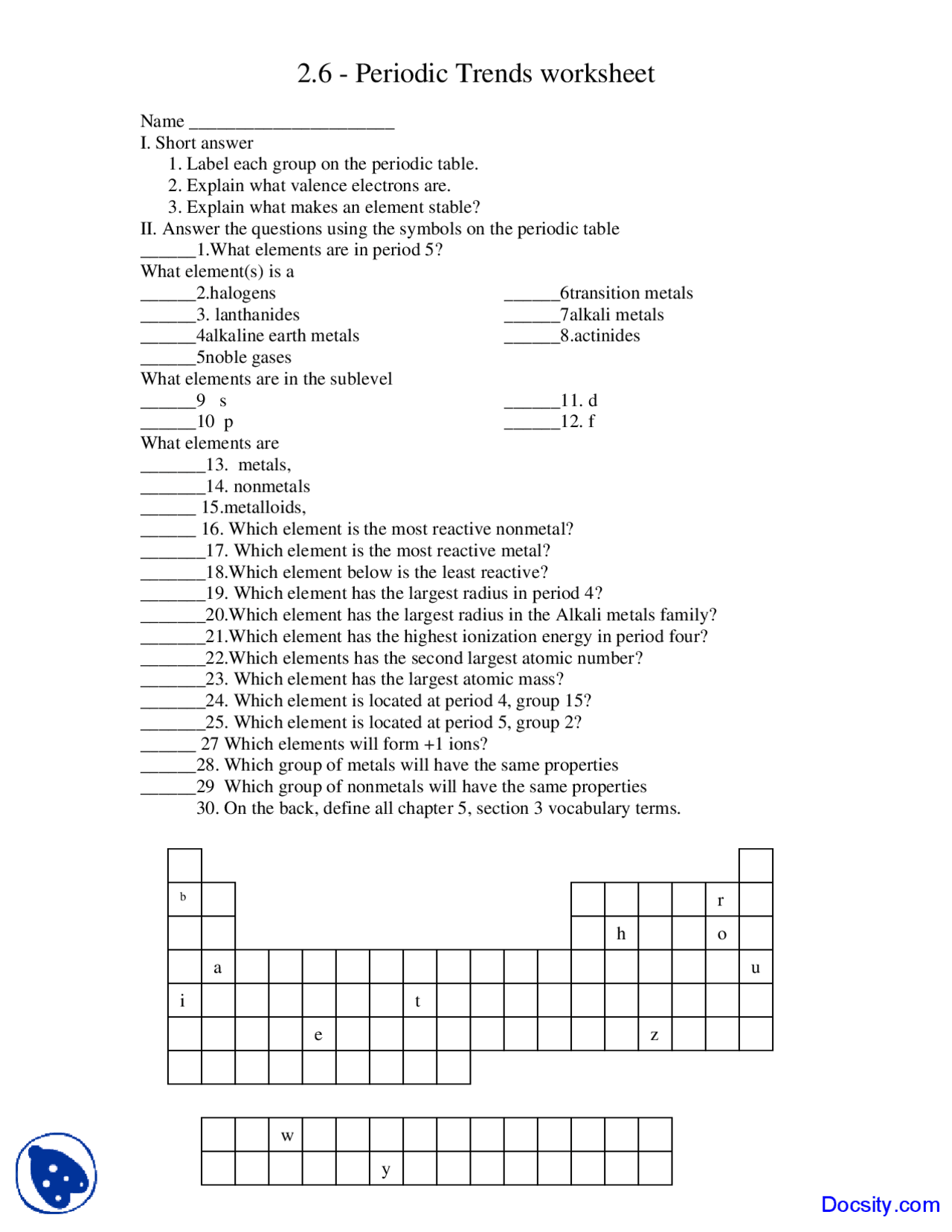
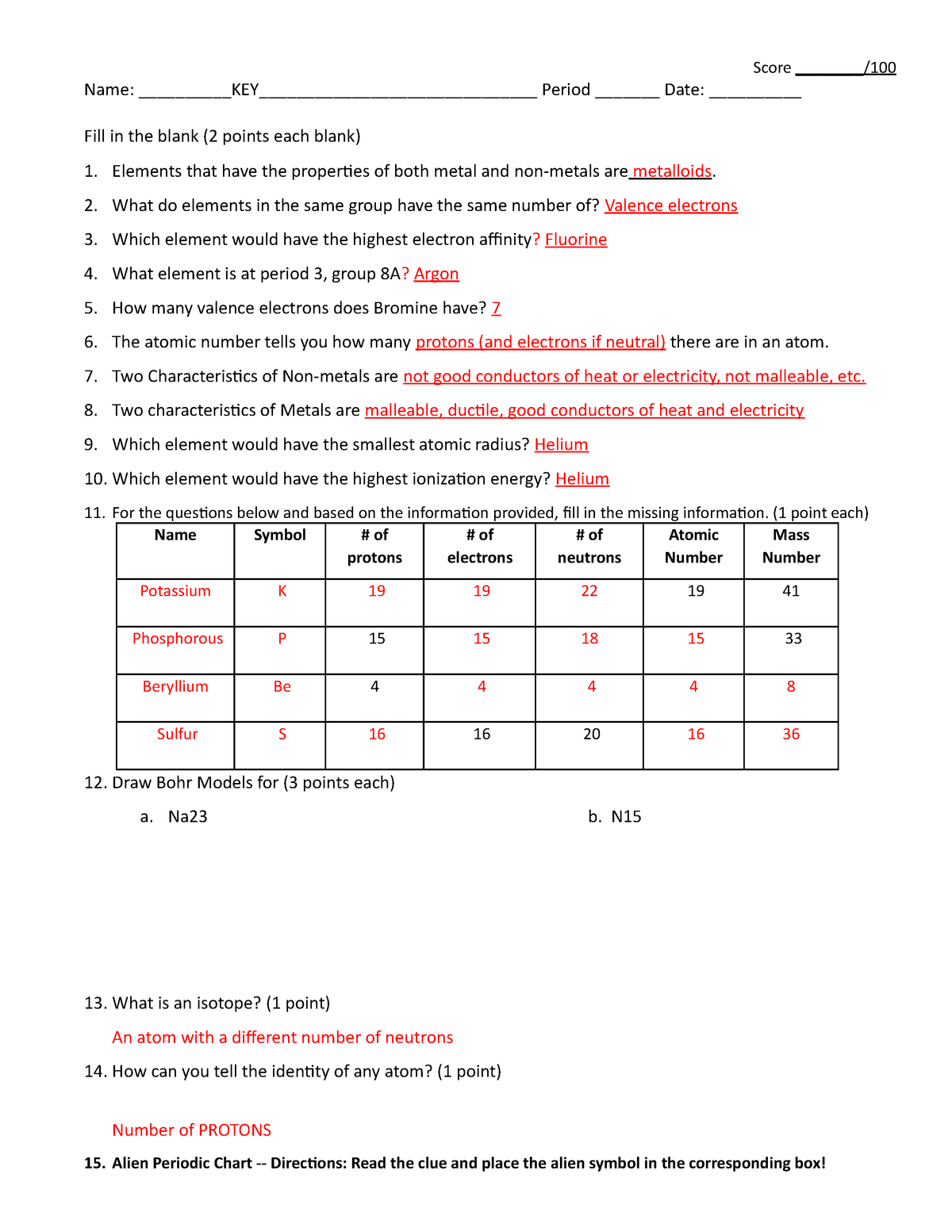
DOC Periodic Trends Worksheet - Winston-Salem/Forsyth County Schools Periodic Trends. For each of the following exercises, explain your answer. Exercises: 1. In each of the following pairs, circle the species with the higher first ionization energy: (a) Li or Cs (b) Cl- or Ar (c) Ca or Br (d) Na+ or Ne (e) B or Be _____ 2. In each of the following pairs, circle the species with the larger atomic radius:
Periodic trends worksheet atomic radius answers
PDF Periodic Trends Worksheet - Martin High School What trends do you notice for the atomic radii of Period 3? 4. Explain why this trend occurs. 5. Ionization energy is the amount of energy required to remove an electron from an ... Periodic Trends Worksheet 1. Using the data below, make a bar graph of atomic radius vs. atomic number for Group 2A and for Period 3 of the periodic table. PDF DCI - Science - Home Created Date: 10/2/2017 3:02:27 PM Solved 1 Periodic Trends Worksheet Kank the following - Chegg Periodic Trends For each of the following exercises, explam your answet ES 1. In each of the following pairs, circle the species with the higher first ionization energy (a) Liors (b) Cor Ar (6) KM B (d) Naor Ne (c) Bor Be 2. In each of the following pairs, circle the species with the larger atomic radius: (a) Mg or Ba (b) S or $2 (e) Cut2 or cu ...
Periodic trends worksheet atomic radius answers. DOC Periodic Trends Worksheet - Socorro Independent School District What trend in atomic radius occurs down a group on the periodic table? What causes this trend? What trend in ionization energy occurs across a period on the periodic table? What causes this trend? Circle the atom in each pair that has the largest atomic radius. Al or B Na or Al S or O O or F Br or Cl Mg or Ca PDF Periodic Trends Worksheet - Currituck County Schools Periodic Trends ATOMIC RADIUS 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? 2. What causes this trend? 3. What trend in atomic radius do you see as you go across a period/row on the periodic table? 4. What causes this trend? 5. Circle the atom in each pair that has the largest atomic radius. PDF Decatur Independent School District / Overview Created Date: 10/18/2018 9:30:29 AM Worksheet Periodic Trends Answer Key - myilibrary.org ANSWER KEY Periodic Trends Worksheet 1. Using the data below, make a bar graph of atomic radius vs. atomic number for Group 2A and for Period 3 of the periodic table. Group 2A Element Atomic Number Atomic Radius Be 4 1.11 Mg 12 1.60 Ca 20 1.97 Sr 38 2.15 Ba 56 2.17 Atomic Number 2. What trends do you notice for the atomic radii of Group 2A?
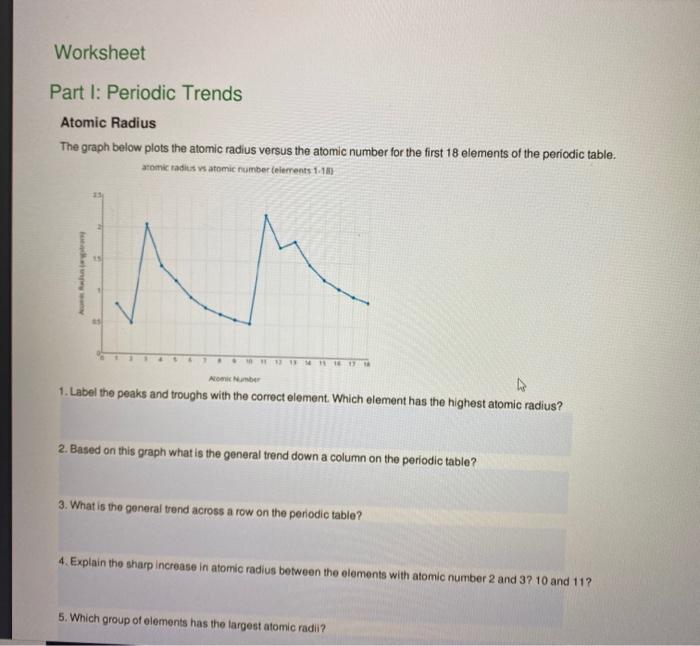
Graphing Periodic Trends Worksheet Answer Key Graphing Periodic Trends - StudyRes. Procedure 1. Define the following terms on a separate sheet of paper: period, group (Family), atomic radius, electronegativity, first ionization energy 2. PDF Worksheet 3.3 Periodic table trends - cpb-ap-se2.wpmucdn.com 2 What trend in atomic radius, if any, is evident in the plot? 3 Suggest a reason for the trend observed. 4 Would you expect the same trend to be observed for the period 2 elements? 5 Sodium is a more reactive metal than magnesium. How does this fact relate to its atomic radius? PDF Livingston Public Schools / LPS Homepage ATOMIC RADIUS For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. a. Li, C, F b. Li, Na, K d. C, N, Al e. Al, Cl, Ga Cli IONIC RADIUS For each of the following sets of ions, rank them from smallest to largest ionic radius. a. Mg , Si b.Mg , ca , Ba c. F, cr, Br d. PDF Periodic Trends Worksheet - luckyscience.com 23) What do you notice about your answers in I vs. your answers in III? Why in this true? Opposite- larger radies equals easier to remove e-IV. Which ion will have the smaller radius? 24) K + or O2-___O2-____ 25) Ba2+ or I-___Ba2+___ 26) Al. 3+ or Cl - ___Cl-____ 27) K + or Ca +2 ___Ca. 2+ ___ 28) P-3. or S. 2-___S. 2-_____ 29) Why is the last ...
Periodic trends worksheet Flashcards | Quizlet What is the trend in size (atomic radius) going down a group on the periodic table and why does this occur? The size increases when the principal quantum number increases (n) What is the trend in sized (atomic radius) within the same period going to the right and why does this occur? What is electronegativity? Attractive force that an atom has ... Periodic Trends Quiz Teaching Resources | Teachers Pay Teachers Periodic Trends Quiz HS PS1-2. by. Mr Waggoner Chemistry Biology NGSS. $1.90. PDF. Quiz for the following learning objective: 1) Students will be able to explain how and why the following periodic trends change across the periodic table: atomic radius, electronegativity, ionization energy, electron affinity. Subjects: PDF Periodic Trends Worksheet - mrsgingrashths.weebly.com Periodic Trends Worksheet 1) Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. 2) Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum. 3) What is the diffe rence between electron affinity and ionization energy? Periodic Trends Worksheet answers.docx - Name Period Date... Get's bigger 2. What causes this trend? Adds energy levels 3. What trend in atomic radius do you see as you go across a period/row on the periodic table? Goes down 4. What causes this trend? Additional pull of more protons 5. Circle the atom in each pair that has the largest atomic radius. a) Al B b) S O c) Br Cl d) Na Al e) O F f) Mg Ca 6.
Solved Periodic Trends Worksheet Trends-Atomic Radii (size ... - Chegg Science; Chemistry; Chemistry questions and answers; Periodic Trends Worksheet Trends-Atomic Radii (size), lonization Energy, Electronegativity 1. Circle the one from each pair that would have the larger atomic radius (larger in size): (A) F atom or Qatom (B) Ba atom on Bantom (cHf atom or Ti atom (D) Ta atom or At atom (E) Cu atom or K atom (F) Np atom or Cf atom (G) Mn atom or Re atom (H) Dy ...
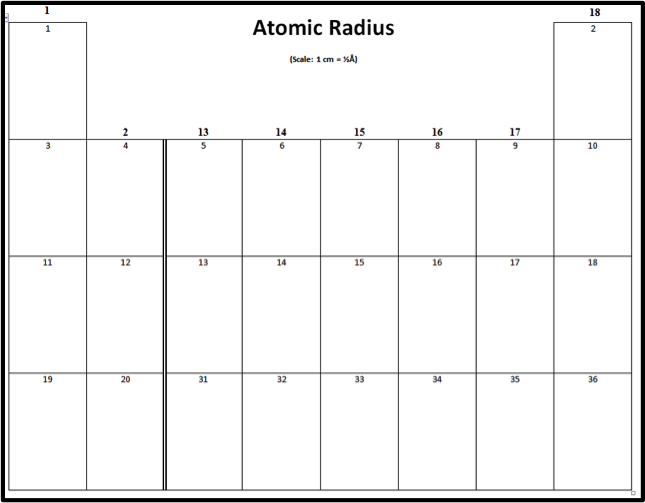
PDF Periodic Trends Worksheet Atomic Radius vs. Atomic Number (Period 3) Periodic Trends Worksheet Atomic Radius 1. Using the data below, make a bar graph of atomic radius vs. atomic number for Group 2A and for ... Radius Be 4 1.11 Mg 12 1.60 Ca 20 1.97 Sr 38 2.15 Ba 56 2.17 Atomic Radius Atomic Number Answer the following questions about atomic radius in complete sentences. Period 3 Element Atomic Number Atomic ...
worksheet periodic trends periodic worksheet trends answers atomic worksheets answer key table doc sponsored links. Matter/Atoms/Elements - Lincoln 8th Grade Science ... periodicity science melting compoundchem organic energy compound point elements properties teaching cheat chart v2 radius ionization. 6 Images Periodic Table Trends Pdf And View - Alqu Blog
PDF WS 3-3 Periodic Trends - mychemistryclass.net Worksheet 3-3 Name Periodic Trends Period 1. Discuss the importance of Mendeleev's periodic law. 2. Identify each element as a metal, metalloid, or nonmetal. ... Circle the atom in each pair that has the largest atomic radius. a) Al B b) S O c) Br Cl d) Na Al e) O F f) Mg Ca 7. Define ionization energy. ...
PDF Periodic trends: Atomic radius answers. Name the atomic radius gets bigger. For a more thorough exploration of atomic radius, download the following articles: Craft, J., and J.S. Miller. 2007. Unlocking the atom. The Science Teacher 74 (2): 24-29. McInerny, W. 1999. Probability and atomic radius in the H atom. Journal of Chemical Education 76 (3): 443-444.
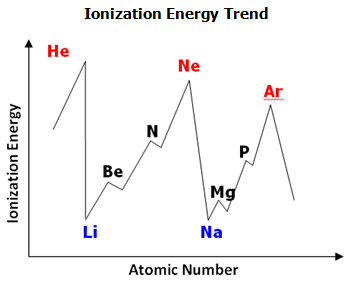
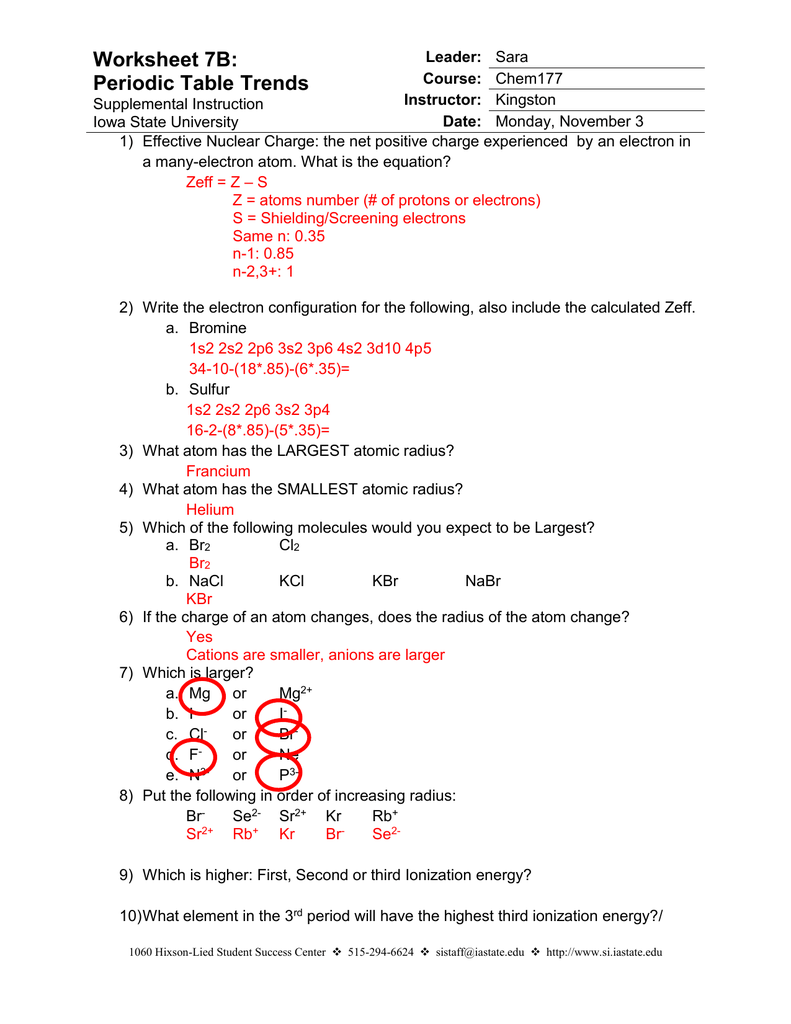
PDF Periodic Trends Worksheet Answers - MS. SWARTZ It increases as you go down the periodic table in a group because you fill more Energy levels. It decreases as you go right across a period because there are more protons in the nucleus (increasing nuclear charge) and the valence electrons are more strongly attracted. 2. Rank the following elements by increasing atomic radius: carbon, aluminum ...
PDF Periodic Trends Worksheet - mayfieldschools.org Worksheet: Periodic Trends 1. ATOMIC RADIUS For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. a. Li, C, F b. Li, Na, K ... Answers for Comparing Atomic Sizes Here are answers for the questions above. a. Li, C, F All are in the same period and thus have the same number of energy levels. Therefore ...
Periodic trends gizmo - Worksheet - BP 723 - Harvard - StuDocu MATH 1201 Discussion Forum Unit 1. Gizmo periodic trends - Lecture notes bio tech college gizmo. Fundamentals of Nursing 9th Edition Taylor Test Bank-1-10. Ati TEST BANK 2019 PROCTURED. 1-2 Module One Activity Project topic exploration. WK Number 2 Atomic Structure Chemistry 1 Worksheet Assignment with answers. The Martian and the Car.
Periodic Trends Worksheet Answers.docx - Course Hero Name Periodic Trends 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? Increases 2. What causes this trend? Number of shell increases 3. What trend in atomic radius do you see as you go across a period/row on the periodic table? decreases4. What causes this trend? more electrons, higher attraction 5.
PDF Worksheet on Periodic Trends Name - mychemistryclass.net 2. Below is a rough sketch of the Periodic Table. Sketch in whether the following increase or decrease going across a period, left to right or going up a group: electronegativity, atomic radius, electron affinity, ionization energy. Use the Periodic Table and your knowledge of periodic trends to answer the following questions. 3.
Periodic Trends Worksheet - Houston Community College Periodic Trends Worksheet. Directions: Use your notes to answer the following questions. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum. Why does fluorine have a higher ionization energy than iodine?
Solved 1 Periodic Trends Worksheet Kank the following - Chegg Periodic Trends For each of the following exercises, explam your answet ES 1. In each of the following pairs, circle the species with the higher first ionization energy (a) Liors (b) Cor Ar (6) KM B (d) Naor Ne (c) Bor Be 2. In each of the following pairs, circle the species with the larger atomic radius: (a) Mg or Ba (b) S or $2 (e) Cut2 or cu ...
PDF DCI - Science - Home Created Date: 10/2/2017 3:02:27 PM
PDF Periodic Trends Worksheet - Martin High School What trends do you notice for the atomic radii of Period 3? 4. Explain why this trend occurs. 5. Ionization energy is the amount of energy required to remove an electron from an ... Periodic Trends Worksheet 1. Using the data below, make a bar graph of atomic radius vs. atomic number for Group 2A and for Period 3 of the periodic table.





















0 Response to "45 periodic trends worksheet atomic radius answers"
Post a Comment