39 calculating average atomic mass worksheet
Calculating Average Atomic Mass Worksheet … Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a … DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculating Average Atomic Mass Worksheet Name_____ Percents need to be in decimal form. 1. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. ... Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a mass of 34. 3. The four isotopes ...
average_atomic_mass (3).docx - Calculating Average … Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu …
Calculating average atomic mass worksheet
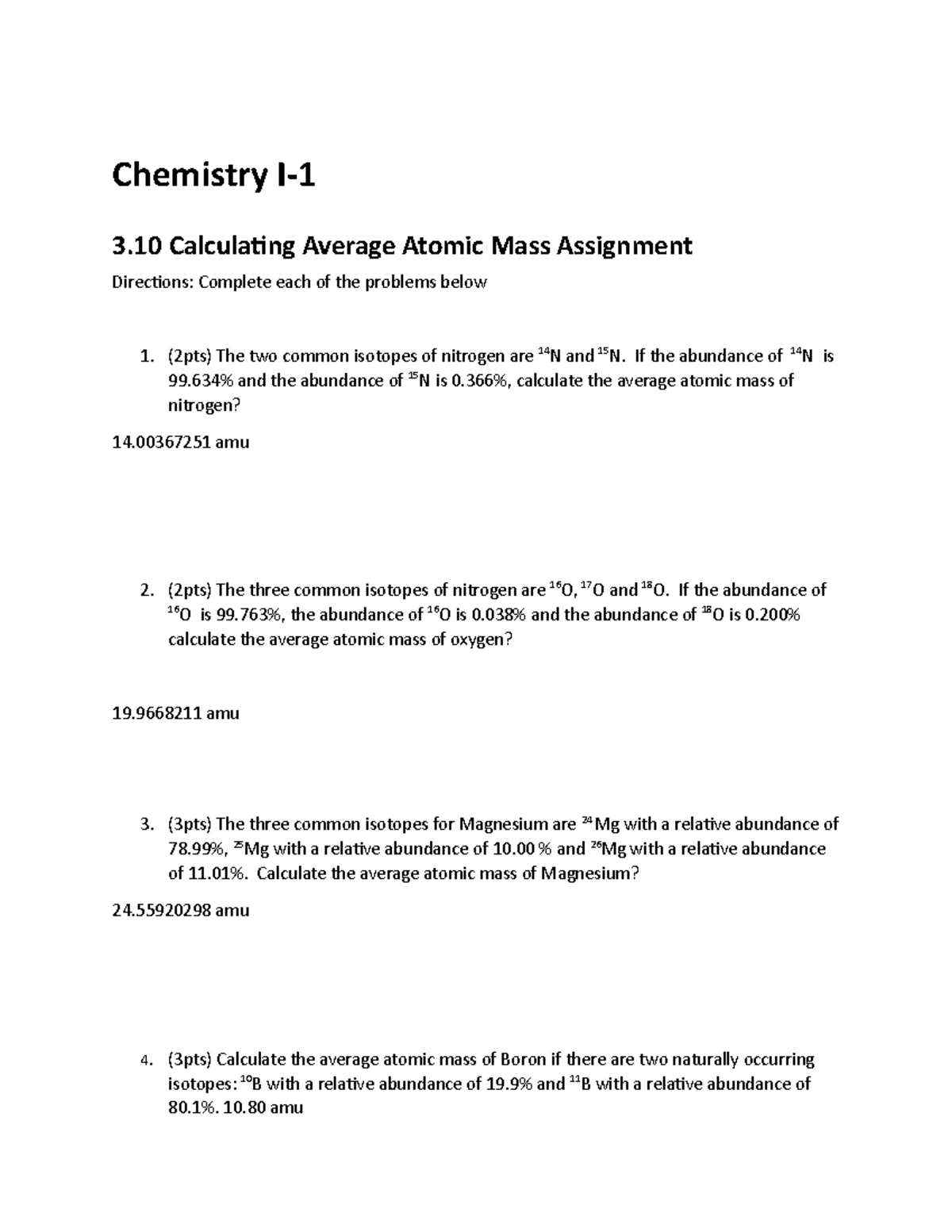
Average_Atomic_Mass.pdf - Calculating Average Atomic Mass Worksheet: 1 ... View Average_Atomic_Mass.pdf from MGMT 101 at Health Services Academy. Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent PDF Calculating Average Atomic Mass Worksheet Name - Solano Community College Calculating Average Atomic Mass Worksheet Name_____ 1. The term "average atomic mass" is a _____average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for ... Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...
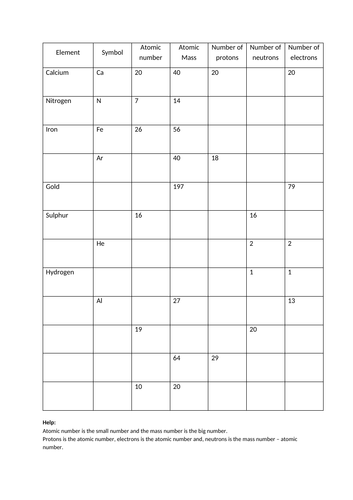
Calculating average atomic mass worksheet. NAME Average Atomic Mass Worksheet: show all work. The average atomic mass of the three isotopes is 24.3050 amu. If the atomic mass of 25Mg is 24.98584 amu, and 26Mg is 25.98259 amu, calculate the actual atomic mass of 24Mg. 24Mg … Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average … Calculating Average Atomic Mass Worksheet Calculating Average Atomic Mass Worksheet Posted on July 27, 2022 by admin Students generally abash diminutive number, diminutive mass, diminutive weight and about diminutive mass. The afterward definitions may be helpful: Atomic cardinal is the cardinal of protons in an element. Calculating Average Atomic mass Worksheet-1 ( 1).docx Oct 21, 2020 · Calculate the average atomic mass of copper.
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit.-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% ... Calculating Average Atomic Mass Worksheet - Key Included Practice calculating average atomic mass with this 12 problem worksheet. Perfect for classwork, homework, extra practice, or as examples for students in a distance learning setting.This product includes the following as a PDF file:12 Problems - Calculating Average Atomic MassAnswer Key ... PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculating Average Atomic Mass Worksheet - Name Calculating Average Atomic Mass Worksheet University The City College of New York Course Physical Chemistry I (CHEM 33000) Academic year 2020/2021 Helpful? Comments Please or …
Average_Atomic_Mass.pdf - Calculating Average Atomic … Calculating Average Atomic Mass Worksheet:1)Three isotopes of Silicon occur in nature:Isotopes of Silicon:Percent Abundance:Atomic Mass:Silicon-2892.23%27.97693 … PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 2) Two isotopes Of ... calculating average atomic mass worksheet (1).doc Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% have a mass of 32.971amu … DOC Chemistry Worksheet - Livingston Public Schools Isotope Atomic mass (amu) Natural abundance (atom %) 28Si 28.0 92.2 29Si 29.0 ? 30Si ?? 3.1 The average atomic mass of silicon is 28.09amu. %29Si = 4.7% mass = 29.4amu Calculate the relative abundance of each isotope of iridium. The average atomic mass of iridium is 192.22amu Isotope mass (u) relative abundance Ir-191 191.0 ? 39.00%
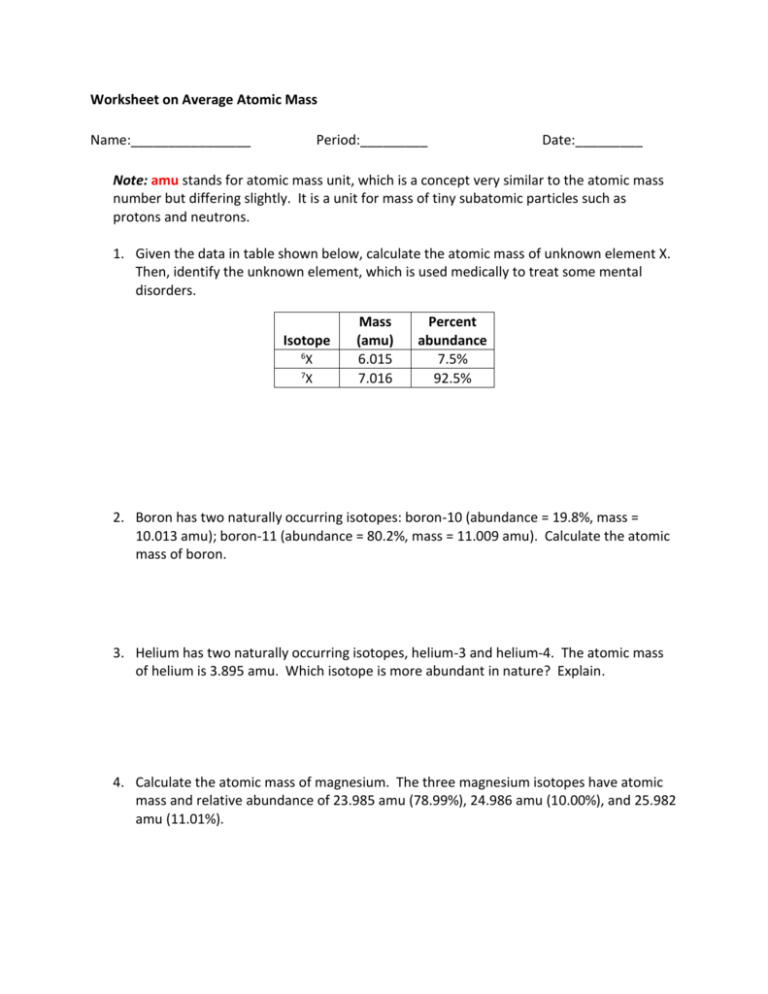
calculating average atomic mass worksheet (1).doc - Name_... 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% have a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%.
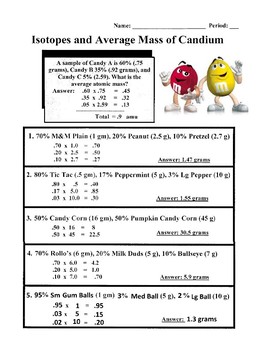
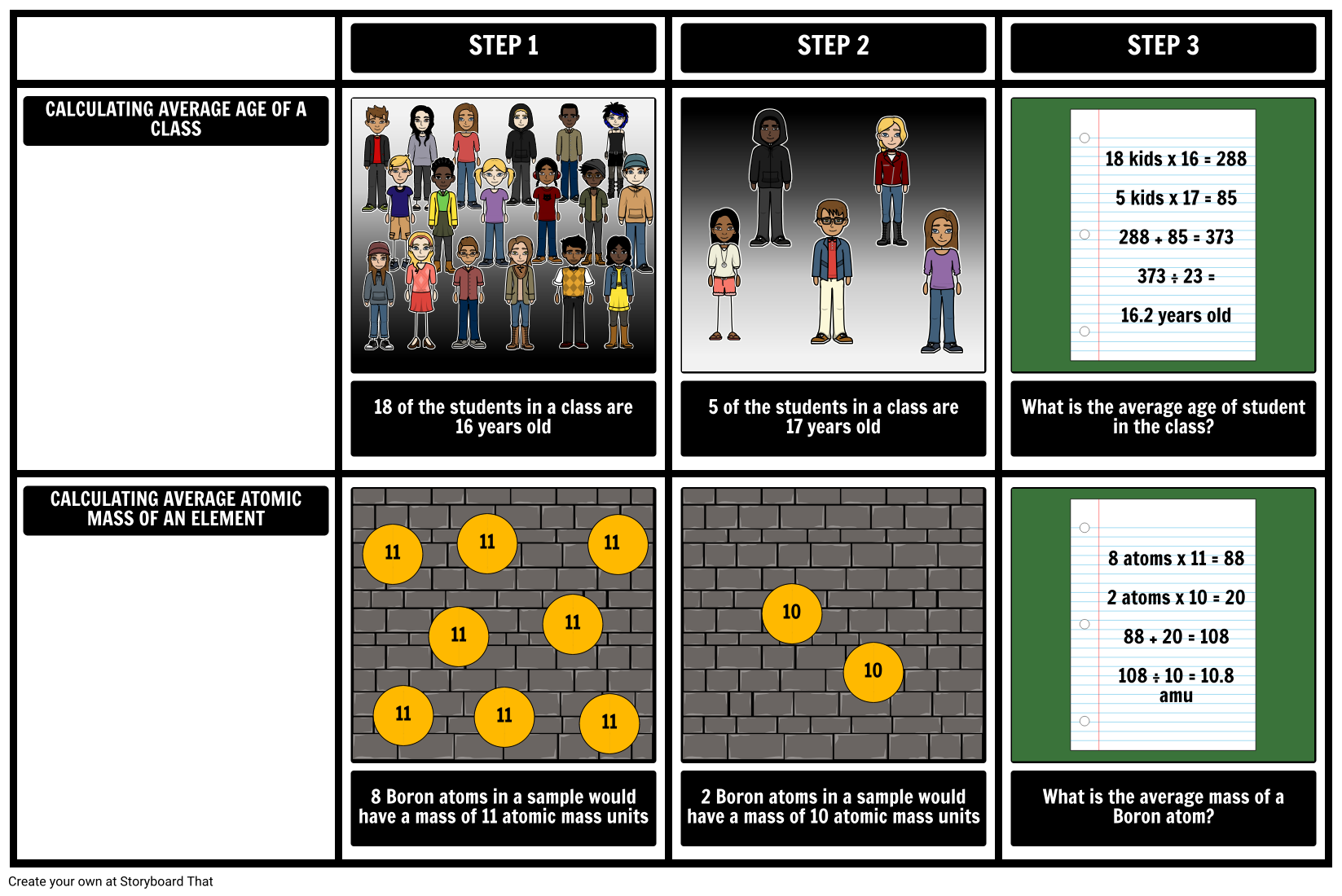
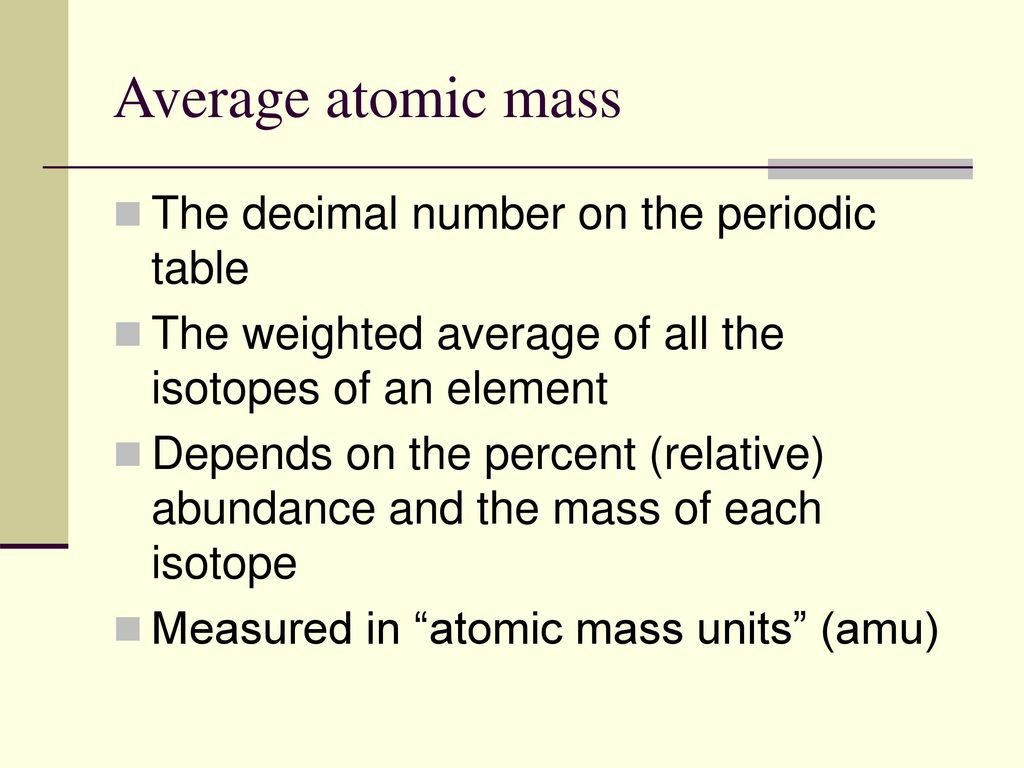
Calculating Average Atomic Mass Worksheet | Aurumscience.com. Calculating Average Atomic Mass Part of understanding isotopes is realizing how their abundance determines the average atomic mass shown with each element of the periodic table. This worksheet will show students how these numbers are calculated, and help them understand why the atomic mass of oxygen is 15.99 AMU instead of simply 16 AMU.
Calculating Average Atomic Mass Worksheet.docx - Name Calculate the average atomic mass of copper. 2.Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 3. What is the average atomic mass of strontium? Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88.
Calculating Average Atomic Mass Worksheet 2022.doc - Mr.... 1. The term "average atomic mass" is a _____average, and so is calculated differently from a "normal" average. Explain how this type of average is calculated. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69.2% for mass of 62.93u 30.8% fora mass of 64 ...
average_atomic_mass (3).docx - Calculating Average Atomic... Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6.
PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ...
Calculating Average Atomic Mass Worksheet Name - Scribd The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4.
Solved Calculating Average Atomic Mass Worksheet: 1) Three - Chegg Chemistry questions and answers. Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
Calculating Average Atomic mass Worksheet-1 ( 1).docx A=(62.93x 69.2\100) + (64.93x 30.8\100) = 43.54 + 19.99 = 63.53 A = 63.53 amu 5.Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. (31.972x 95.00\100) + ( 32.971x 0.76 /100) + ( 33.967x 4.22/100) 30.3734 + 0.250 + 1.433 A= 32.056 amu 6.
Calculating Average Atomic Mass Worksheet - Name … 69/100 x 62 = 43.30/100 x 64 = 19.ggghvhvhvhvhhv= 63. Calculate the average atomic mass of Sulfurif:95% of all Sulfur atoms have a mass of 31 u,0% has a mass of 32and 4% have a …
Calculating Average Atomic Mass Worksheet | Aurumscience.com. Calculating Average Atomic Mass Part of understanding isotopes is realizing how their abundance determines the average atomic mass shown with each element of the periodic …
Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...
PDF Calculating Average Atomic Mass Worksheet Name - Solano Community College Calculating Average Atomic Mass Worksheet Name_____ 1. The term "average atomic mass" is a _____average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for ...
Average_Atomic_Mass.pdf - Calculating Average Atomic Mass Worksheet: 1 ... View Average_Atomic_Mass.pdf from MGMT 101 at Health Services Academy. Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent


























0 Response to "39 calculating average atomic mass worksheet"
Post a Comment