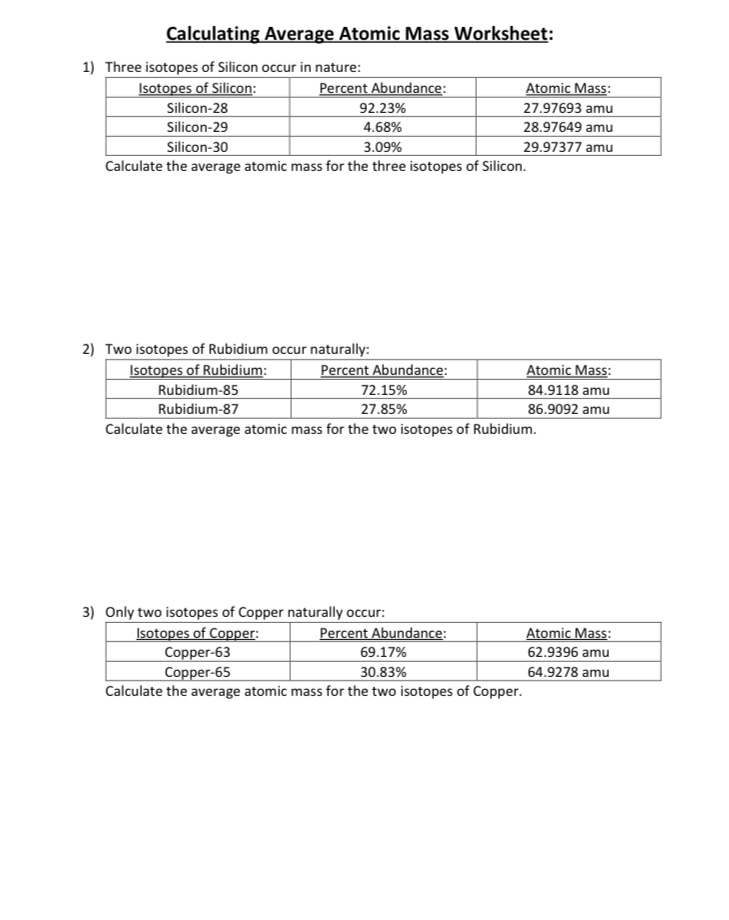
39 isotopes and average atomic mass worksheet
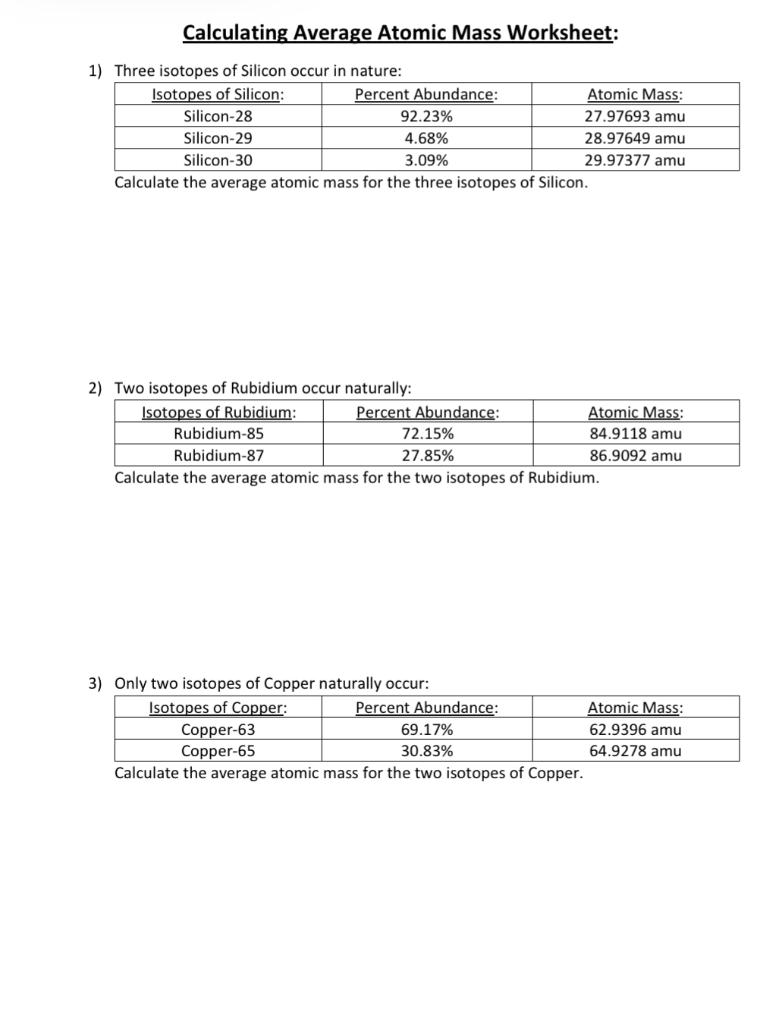
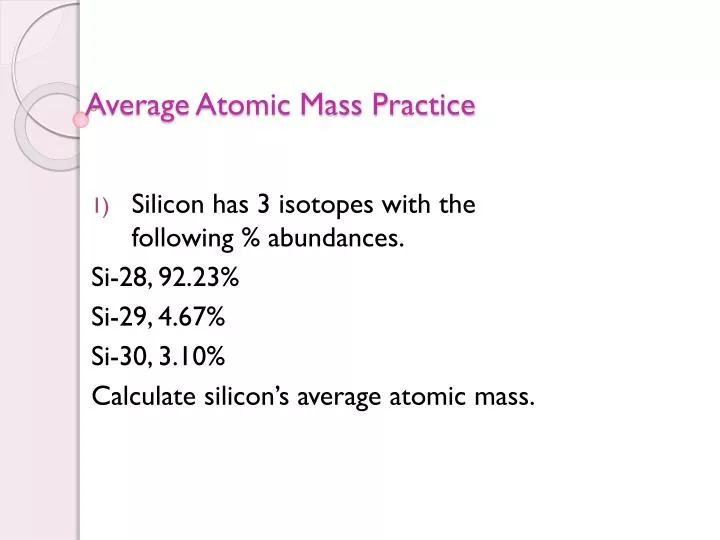
› userfiles › 2856Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and › cms › lib04NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu.
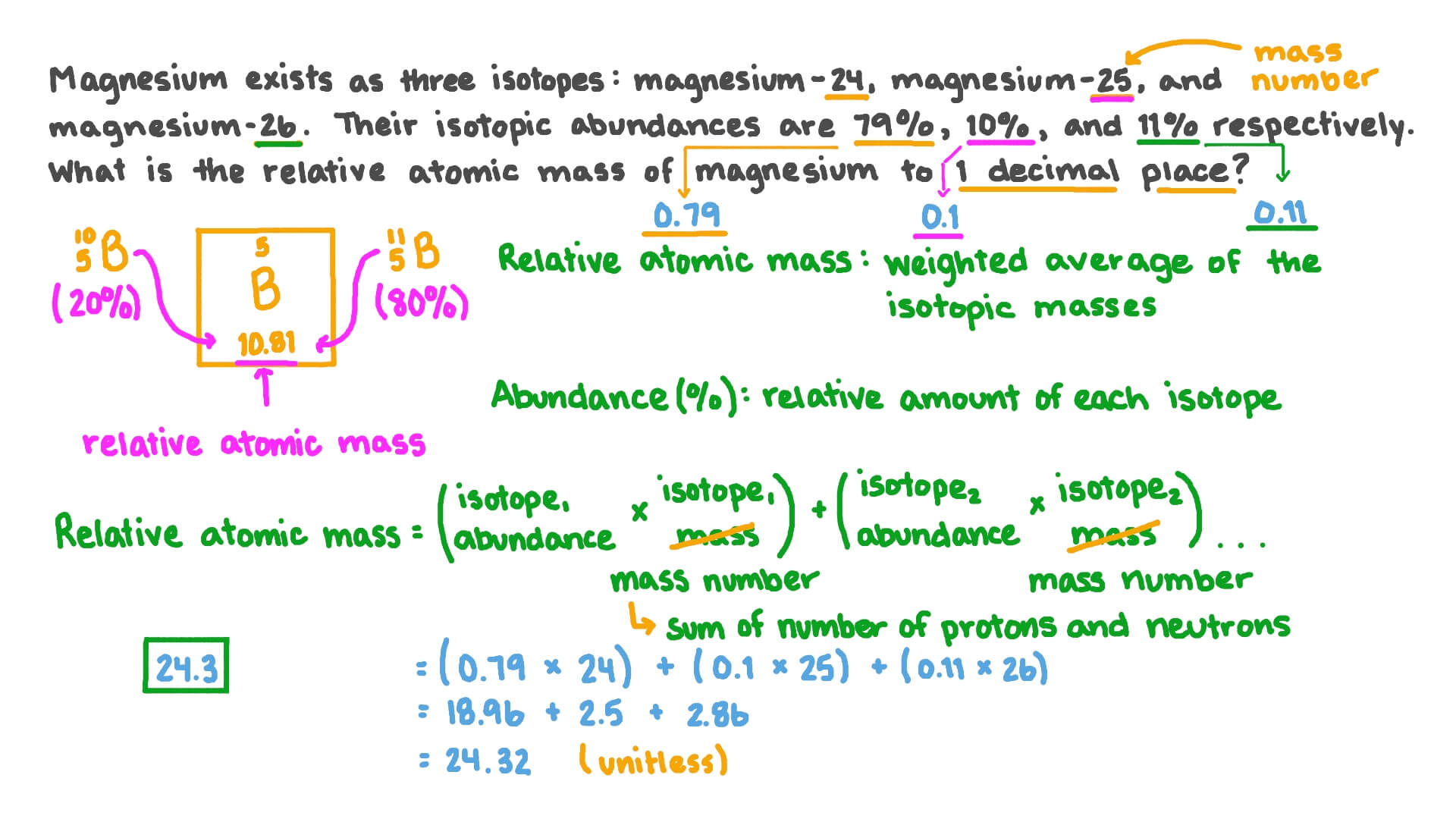
› cms › libAtomic Structure Worksheet - Washoe County School District The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the atomic number. The of an element is the average mass of an element’s naturally occurring atoms, or isotopes, taking into account the of each isotope.

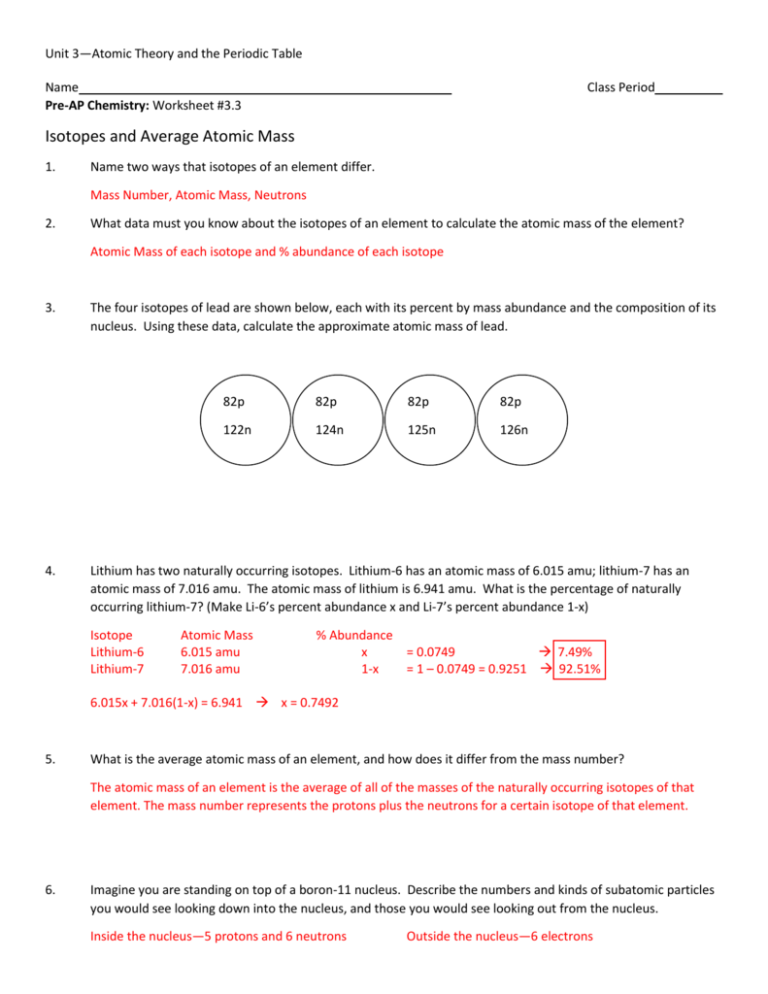
Isotopes and average atomic mass worksheet
chem.libretexts.org › Courses › Oregon_Institute_of2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments. | ExploreLearning Solve the math fact fluency problem. Adaptive and individualized, Reflex is the most effective and fun system for mastering basic facts in addition, subtraction, multiplication and division for grades 2+. study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element?
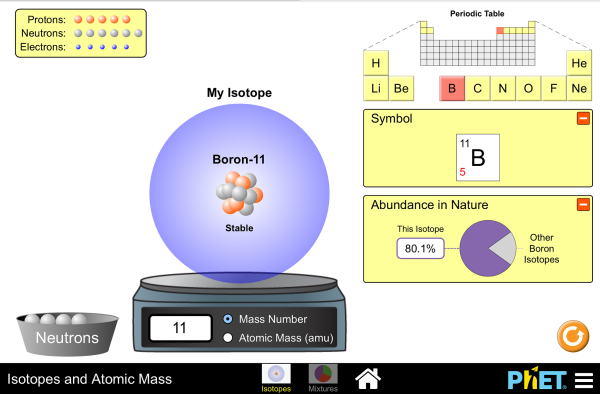
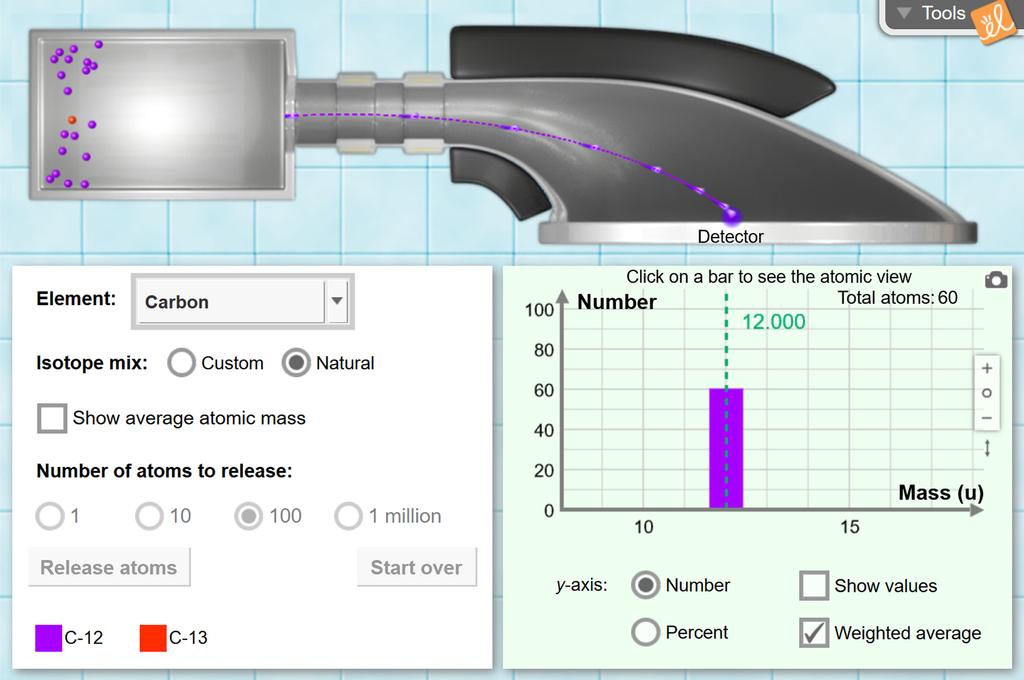
Isotopes and average atomic mass worksheet. phet.colorado.edu › sims › htmlIsotopes and Atomic Mass - PhET Isotopes and Atomic Mass - PhET study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? | ExploreLearning Solve the math fact fluency problem. Adaptive and individualized, Reflex is the most effective and fun system for mastering basic facts in addition, subtraction, multiplication and division for grades 2+. chem.libretexts.org › Courses › Oregon_Institute_of2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments.
















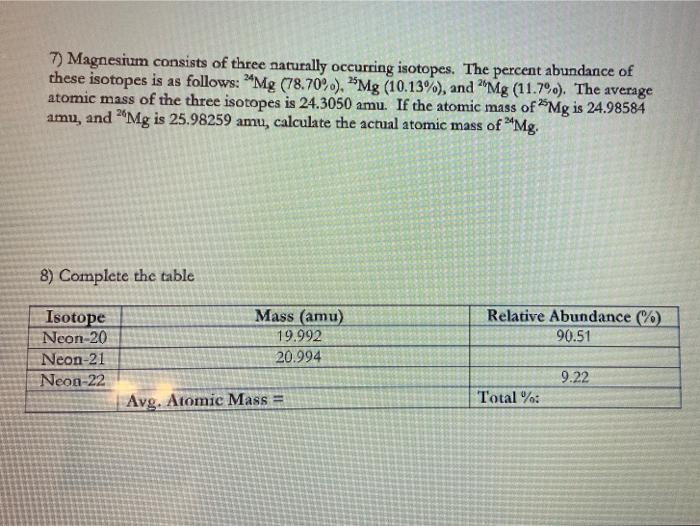


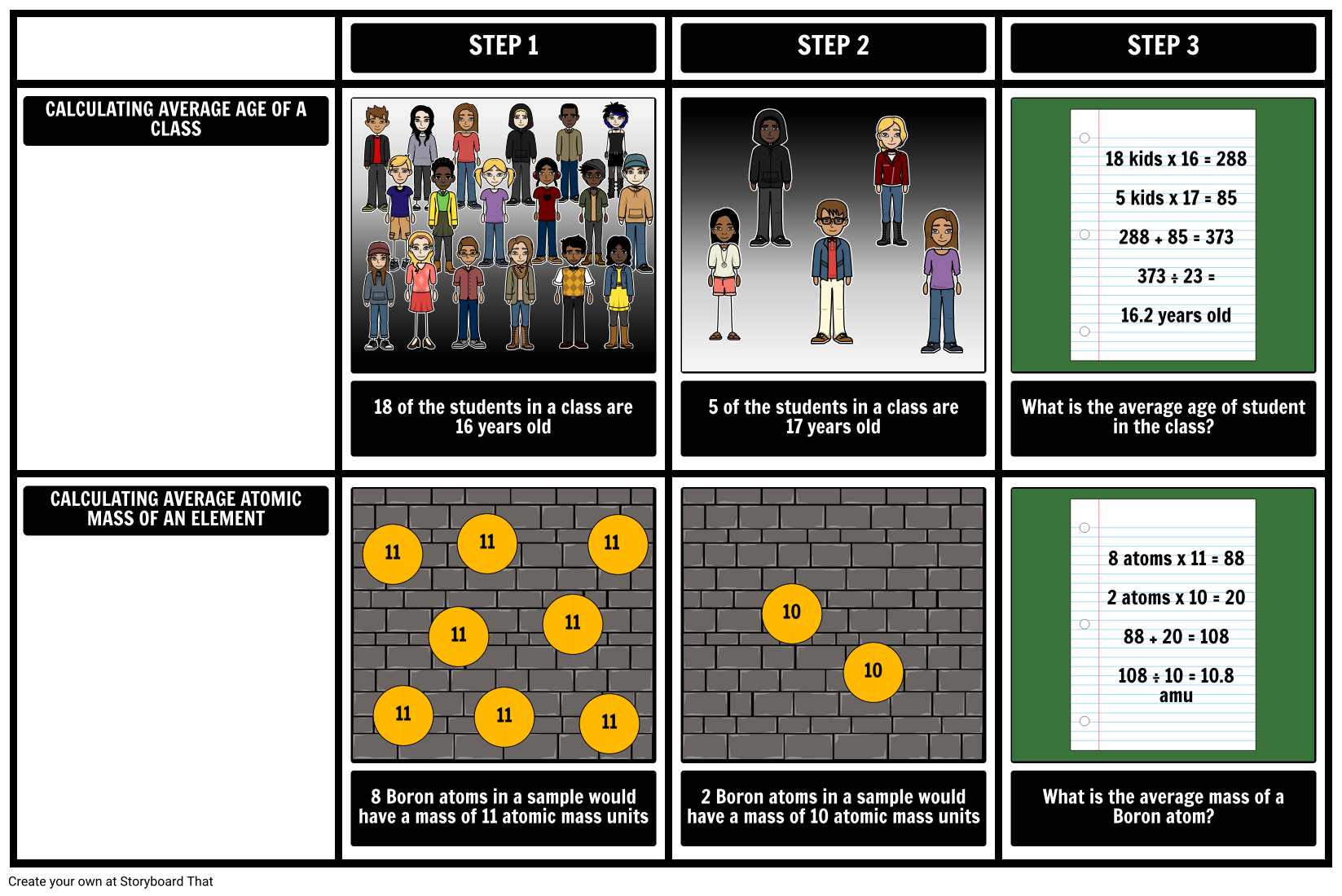



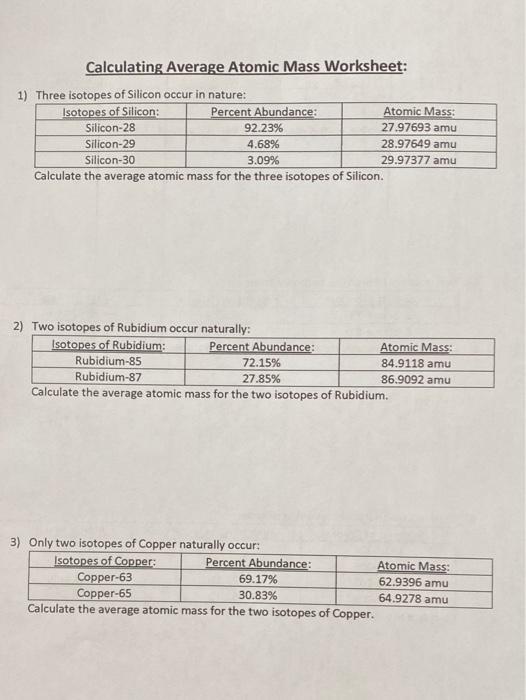



0 Response to "39 isotopes and average atomic mass worksheet"
Post a Comment