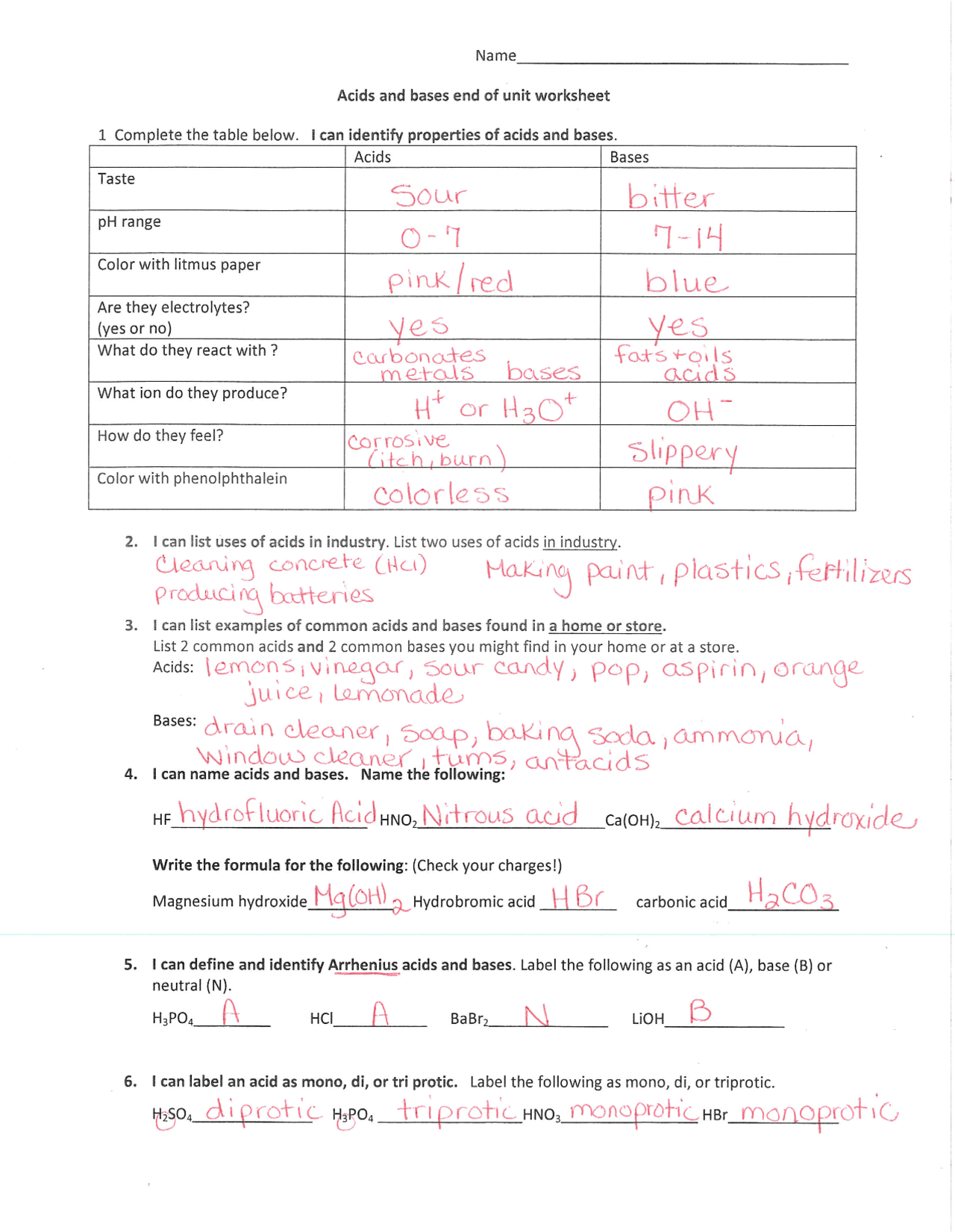
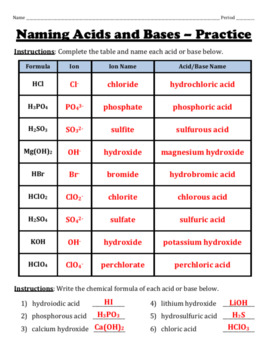
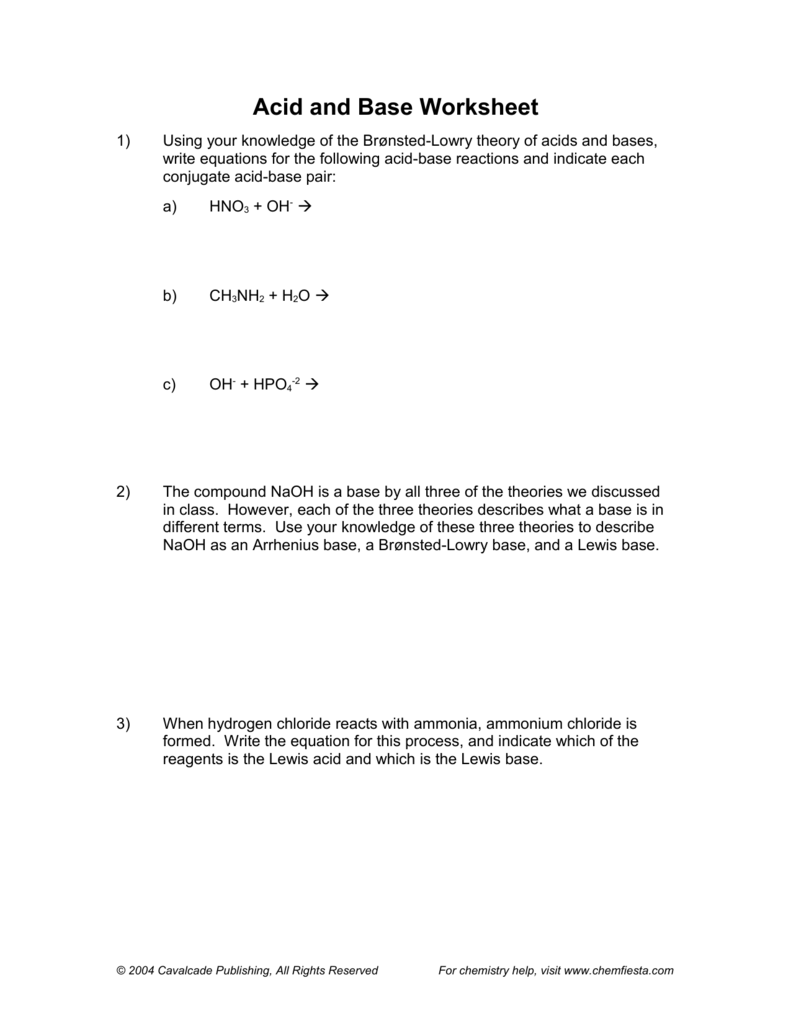
43 chemistry acids and bases worksheet
› high_school_chemistry-helpCalculating pH and pOH - High School Chemistry - Varsity Tutors It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction. All of the bases proceed in a similar fashion. openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - Chemistry 2e ... An earlier chapter of this text introduced the use of element symbols to represent individual atoms. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species.
› Chem12 › chem12Chemistry 12 some review of unit 4-2 (acids, bases and salts) common acids and bases - worksheet 4-1 keyp1 p2. bronsted - lowry theory of acids and bases and acid base equilibria - worksheet 4-2 worksheet 4-2 key_p1 _p2 _p3 _p4 _p5 _p6. ph, poh and kw calculations and problems - worksheet 4-3 worksheet4-3 key p_1 p_2

Chemistry acids and bases worksheet
› study-guides › chemistryFreezing and Boiling Points - CliffsNotes Acids and Bases. Quiz: The pH Scale; Strong and Weak Acids; Quiz: Strong and Weak Acids; Two Types of Bases; Quiz: Two Types of Bases; Polyprotic Acids; Quiz: Polyprotic Acids; Introduction to Acids and Bases; Quiz: Introduction to Acids and Bases; The pH Scale; Oxidation‐Reduction Reactions. Introduction to Oxidation-Reduction Reactions byjus.com › jee › bronsted-lowry-theoryBronsted-Lowry Theory - Definition of acid and base and ... Summary: A Bronsted-Lowry acid is a substance which donates a proton or H + ion to the other compound and forms a conjugated base.; A Bronsted-Lowry base is a substance which accepts a proton or H + ion from the other compound and forms conjugated acid. › how-to-calculate-ph-quickHere's How to Calculate pH Values - ThoughtCo May 02, 2020 · Review of Acids and Bases . There are several ways to define acids and bases, but pH specifically only refers to hydrogen ion concentration and is applied to aqueous (water-based) solutions. When water dissociates, it yields a hydrogen ion and a hydroxide. See this chemical equation below.
Chemistry acids and bases worksheet. › cbse › important-questions-classCBSE Class 12 Chemistry Important Questions 2022-23 - VEDANTU The subject-matter experts at Vedantu have prepared these questions with close reference to the CBSE Class 12 Chemistry latest syllabus, sample papers, previous year question papers. The Class 12 Chemistry important questions is an ultimate guide for the preparation of the class 12 board exams. Students will get a clear idea about the topics ... › how-to-calculate-ph-quickHere's How to Calculate pH Values - ThoughtCo May 02, 2020 · Review of Acids and Bases . There are several ways to define acids and bases, but pH specifically only refers to hydrogen ion concentration and is applied to aqueous (water-based) solutions. When water dissociates, it yields a hydrogen ion and a hydroxide. See this chemical equation below. byjus.com › jee › bronsted-lowry-theoryBronsted-Lowry Theory - Definition of acid and base and ... Summary: A Bronsted-Lowry acid is a substance which donates a proton or H + ion to the other compound and forms a conjugated base.; A Bronsted-Lowry base is a substance which accepts a proton or H + ion from the other compound and forms conjugated acid. › study-guides › chemistryFreezing and Boiling Points - CliffsNotes Acids and Bases. Quiz: The pH Scale; Strong and Weak Acids; Quiz: Strong and Weak Acids; Two Types of Bases; Quiz: Two Types of Bases; Polyprotic Acids; Quiz: Polyprotic Acids; Introduction to Acids and Bases; Quiz: Introduction to Acids and Bases; The pH Scale; Oxidation‐Reduction Reactions. Introduction to Oxidation-Reduction Reactions



























0 Response to "43 chemistry acids and bases worksheet"
Post a Comment