39 redox reactions practice worksheet
Practice: Redox reaction. This is the currently selected item. Practice: Oxidising and reducing agents. Next lesson. Overview on chemical reactions. Redox reaction. Oxidising and reducing agents. Up Next. Oxidising and reducing agents. Our mission is to provide a free, world-class education to anyone, anywhere.
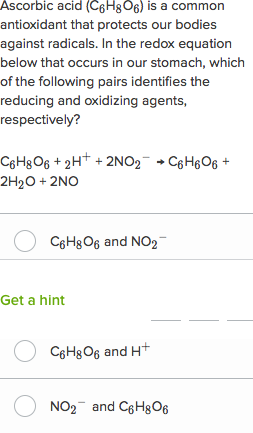
Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 K2CO3 K +1 C 4 O -2 c. NO3- N____+5_____ O____-2_____ HIO4 H +1 I +7 O -2 For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of ...
Questions pertaining to redox reactions If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Redox reactions practice worksheet
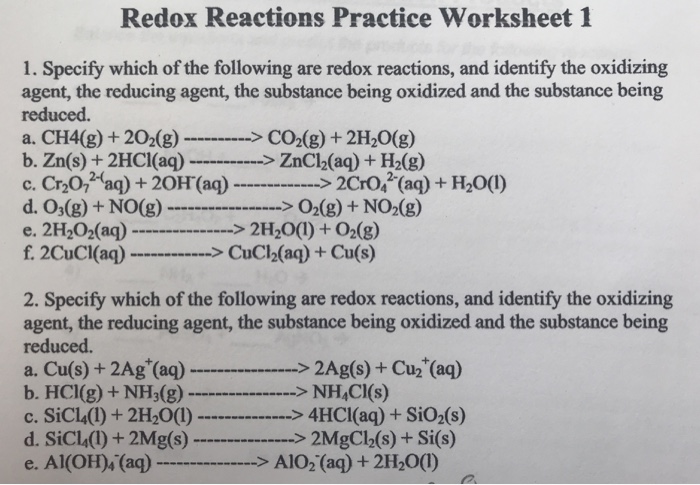
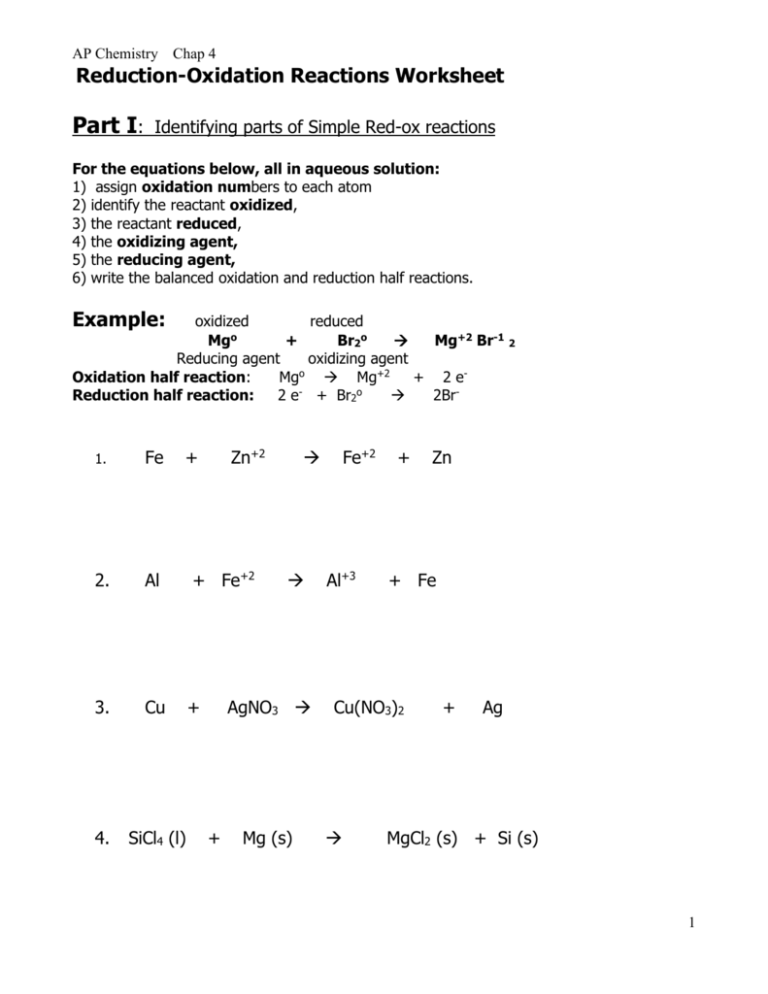
Oxidation-Reduction Worksheet. For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO. 3) 2KMnO4 + 5KNO2 + 3H2SO4 ( 2MnSO4 + 3H2O + 5KNO3 + K2SO4.
This is a single 2-page worksheet with problems that provide students practice balancing redox equations and solving redox titration problems. There are a total of 4 problems. Students will balance 6 half-reactions, and 5 full-reactions. Answer key is included.The download includes a handout master.
admin November 18, 2019. Some of the worksheets below are Redox Reactions Worksheets, useful trick to help identify oxidation and reduction, step by step guide to balance any Redox Equations, explanation of Oxidation, reduction, oxidizing agent, reducing agent and rules for assigning an oxidation number, …. Once you find your worksheet (s ...
Redox reactions practice worksheet.
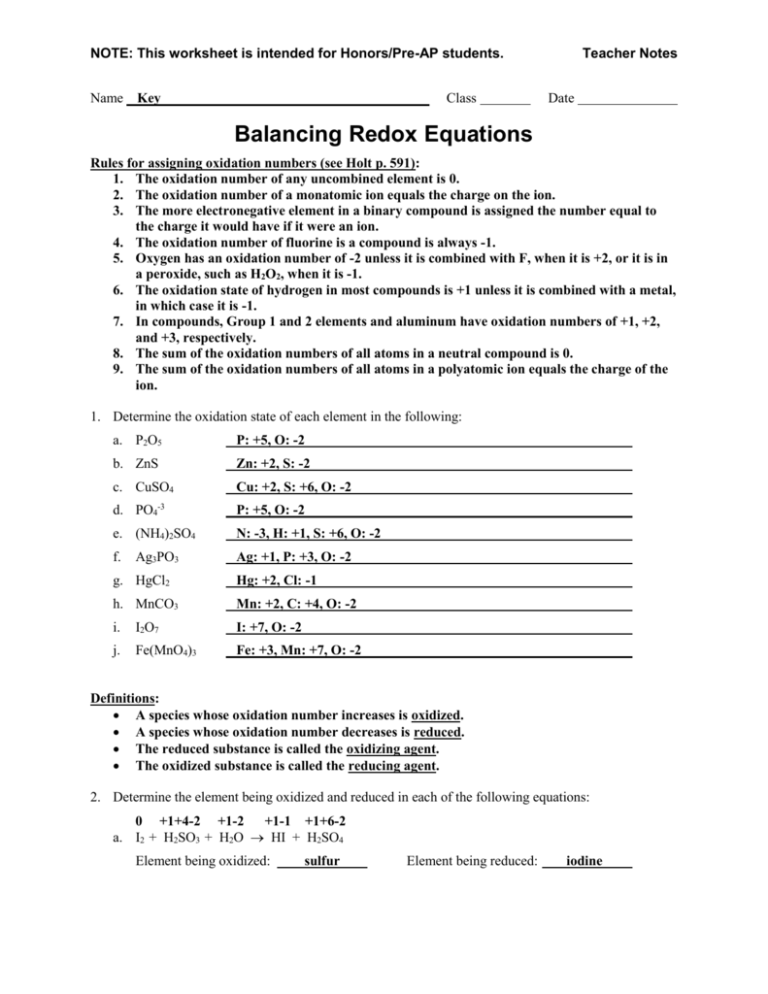
Balancing Redox Equations WorkSheet. Oxidation Number Method for Balancing Redox Equations. 1. Assign oxidation numbers to all elements and identify those ...6 pages
Once you find your worksheets you can either click on the pop-out icon or download button to print. 2Al 3I2 synthesis 2Al 3I2 2AlI3 3. Pin By Saitech Informatics On Balancing Of Chemical Reaction Redox Reactions Chemistry Worksheets Chemistry Education Types of chemical reaction worksheet. Chemical reactions practice worksheet. For each of the following equations. […]
Balancing Redox Reactions Practice Worksheet Key 6 Aq. Chemistry 30 7 3 Balancing Equations With Oxidation. Redox Balancing Worksheet Strongsville City ...
Download printable Chemistry Class 11 Worksheets in pdf format, CBSE Class 11 Balancing of Redox Reactions Worksheet A has been prepared as per the latest syllabus and exam pattern issued by CBSE, NCERT and KVS. Also download free pdf Chemistry Class 11 Assignments and practice them daily to get better marks in tests and exams for Grade 11. Free chapter wise worksheets with answers have been ...
REDOX: Balancing REDOX Reactions Practice Worksheet In this hands on worksheet, students will be reviewing many of the concepts taught in the REDOX unit. The overall goal of each question is to balance the reaction the REDOX way. Not only will students be practicing balancing REDOX reactions, they
Worksheet Redox Half Reactions Practice With Answers. by Hedvig on December 5, 2021 December 5, 2021 Leave a Comment on Redox Half Reactions Practice With Answers. Ncert Solutions For Class 11 Chemistry Chapter 8 Redox Reactions Cbse Tuts Redoxreactionclass11 Redox Reactions 11th Chemistry Chemistry .
Balancing Redox Equations Method 2: Half-reaction method 1. Divide the skeleton reaction into two half-reactions, each of which contains the oxidized and reduced forms of one of the species 2. Balance the atoms and charges in each half-reaction - Atoms are balanced in order: atoms other than O and H, then O, then H
Add the following half-reactions together to determine the overall ionic form of the redox reaction. Label the reduction and oxidation half-reactions and make ...12 pages
Redox reactions practice problems determining oxidation numbers worksheet answers. For example the oxidation number of the oxygen in the oxide ion o 2 is 2. 1 This problem poses interesting problems especially with the Cl. Fe HCl —. In which substance is the oxidation number of. The oxidation number of hydrogen in a compound is 1 4.
Balancing Redox Reactions Worksheet 1 Balance each redox reaction in . acid. solution. Mn 2+ + BiO3 -Æ MnO4 -+ Bi 3+ MnO4 -+ S2O3 2- Æ S4O6 2- + Mn 2+
A Guide to Redox Reaction Teaching Approach In this series we explain reduction-oxidation reactions, called redox for short. ... important that the students learn these rules and then do lots of practice to ensure that they ... Design a worksheet or set of questions about one video lesson. Then ask learners to
Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Balancing redox reactions worksheet 1 balance each redox reaction in. In each case assume that the reaction takes place in an acidic solution also state whether the reaction is oxidation or reduction. In which substance is the oxidation number of nitrogen zero. 2ca o2 2cao 9. There are many methods available for balancing redox reactions a ...
Redox reactions answer key determine the oxidation number of the elements in each of the following compounds. In the reaction 2k cl2 2kcl the species. Balance the reaction and indicate which reactant is oxidized and which reactant is being reduced. Identify the species being oxidized and reduced in each of the. Redox practice worksheet name.
Balancing REDOX Reactions: Learn and Practice Reduction-Oxidation reactions (or REDOX reactions) occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge. We can "see" these changes if we assign oxidation numbers to the reactants and products.
Worksheet # 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution. 4. Cr 2O 7 2 - → Cr3+ 5. NO → NO 3-6. SO 4 2- → SO 2 7. MnO 2 → Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. 8. H 2O 2 + Cr 2O 7 2- → O 2 + Cr 3+ 9. TeO 3 2-+ N 2O 4 → Te + NO 3-10 ...
Redox reactions worksheet gcse
A redox reaction can easily be explained as: an attraction between opposite charges forming a bond by sharing electrons transferring electrons between reactants. the breakdown of glucose in cells ...
Write balanced equations for the following redox reactions: a. 2 NaBr + Cl 2 2 NaCl + Br 2 b. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 in acidic solution c. 5 CO + I 2 O 5 5 CO 2 + I 2 in basic solution ; Write balanced equations for the following reactions: a. Cr(OH) 3 + Br 2 CrO 4 2-+ Br-in basic solution 10 OH-+ 2 Cr(OH) 3 + 3 Br 2 2 CrO 4 2-+ 8 H 2 O ...
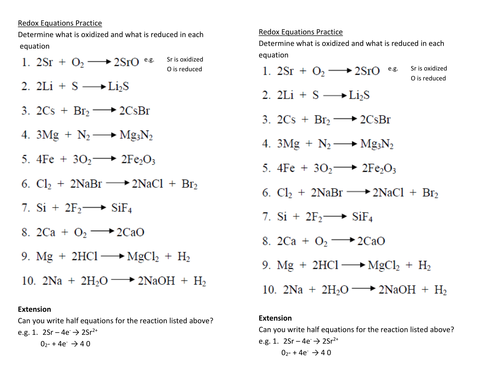
Predicting redox reactions using the half reaction table 1. Redox reactions worksheet. Cr 2o 7 2 cr3 5. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. In the reaction 2k cl2 2kcl the species. In the reaction al0 cr3 al3 cr0 the reducing agent is a. Ws 4 balancing redox reactions. 3mg n2 mg3n2 5. 2cs br2 2csbr 4. 5 2 customer reviews.
Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-d. NO 2-e. LiH f. Fe 3 O 4 Hint; Identify the species being oxidized and reduced in each of the following reactions: a. Cr + + Sn 4+ Cr 3+ + Sn 2+ b. 3 Hg 2+ + 2 Fe (s) 3 Hg 2 + 2 Fe 3+ c. 2 As ...
Chapter 20 Worksheet: Redox I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO 2. 2Li + S Li2S 3. 2Cs + Br2 2CsBr 4. 3Mg + N2 Mg3N2 5. 4Fe + 3O2 2Fe2O3 6. Cl2 + 2NaBr 2NaCl + Br2 7. Si + 2F2 SiF4 8. 2Ca + O2 2CaO 9.
Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Practice exercises. Balance the following equations of redox reactions: Assign oxidation numbers to all elements in the reaction. Separate the redox reaction into two half reactions. Balance the atoms in each half reaction. Add the two half-reactions together and cancel out common terms.
3. Balance the spontaneous redox reaction below. A spontaneous reaction is a reaction that occurs: 1) by a driving force that favors the product, 2) the free energy of the product is lower than the free energy of the reactant, and/or 3) occurs without any outside 'help' such as electrolysis. Identify the entities reduced and oxidized.
Redox practice worksheet Name: Date: 1. In which substance is the oxidation number of nitrogen zero? A. NH3 B. N2 C. NO2 D. N2O 2. What is the oxidation number of carbon in NaHCO3? A. +6 B. +2 C. 4 D. +4 3. In the reaction Al0 +Cr3+!Al3 +Cr0, the reducing agent is A. Al0 B. Cr3+ C. Al3+ D. Cr0 4. In the reaction 2K+Cl2!2KCl, the species ...































0 Response to "39 redox reactions practice worksheet"
Post a Comment