43 gay lussac's law worksheet
Gay-Lussac's law defines the direct relationship between temperature and pressure. When the parameters of a system change, Gay-Lussac's law helps us anticipate the effect the changes have on pressure and temperature. Boyle's law relates pressure and volume: Charles's law relates temperature and volume:
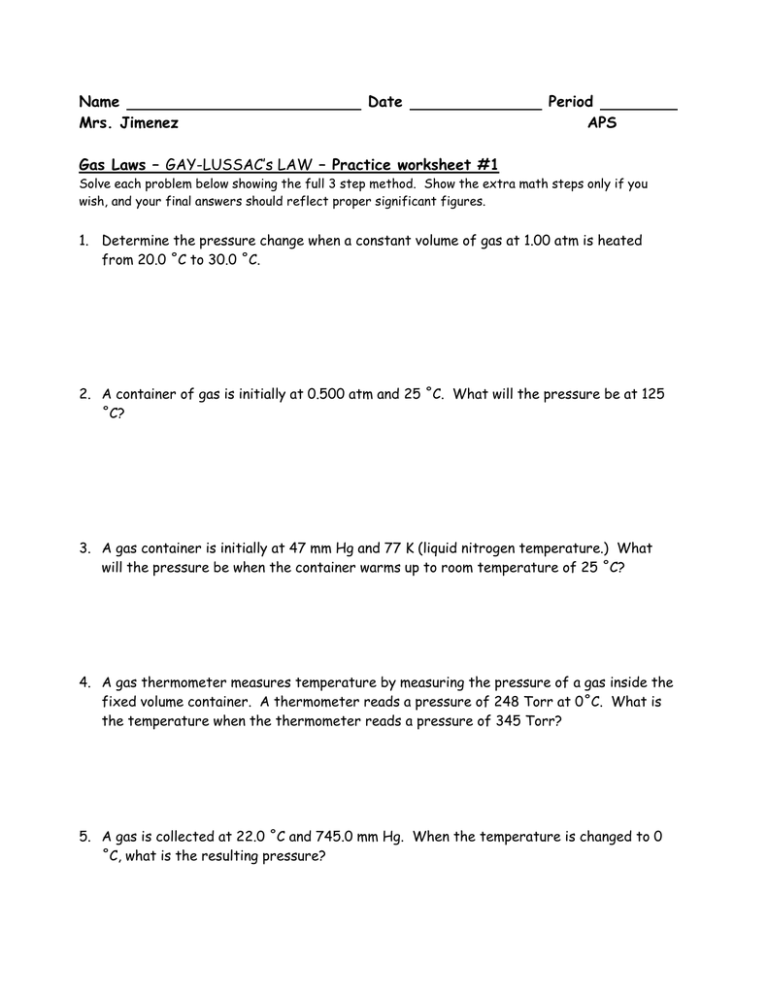
Gay-Lussac's Law Worksheet With Answers1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 ˚C to 30.0 ˚C. 1.03 atm 2. A container of gas is initially at 0.500 atm and 25 ˚C. What will the pressure be at 125 ˚C? 0.668 atm 3. A gas container is initially at 47 mm Hg and 77 K (liquid nitrogen temperature.)
In this worksheet, we will practice using the formula P/T = constant (Gay-Lussac's law) to calculate the pressure or temperature of a gas that is heated or ...

Gay lussac's law worksheet
GAY‐LUSSAC'S LAW WORKSHEET É - Í - L É . Í . L G if 8, J are constant 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 °C to 30.0 °C. 2. A gas has a pressure of 0.370 atm at 50.0 °C. What is the pressure at standard temperature? 3.
Download as DOC, PDF, TXT or read online from Scribd. Flag for inappropriate content. Download now. Save Save Gay Lussac_s Law Worksheet (1).doc For Later. 100% (1) 100% found this document useful (1 vote) 6K views 2 pages.
Gay-Lussac's Law Problems #1 - 10. Ten Examples. KMT & Gas Laws Menu. Problem #1: A 30.0 L sample of nitrogen inside a rigid, metal container at 20.0 °C is placed inside an oven whose temperature is 50.0 °C. The pressure inside the container at 20.0 °C was at 3.00 atm. What is the pressure of the nitrogen after its temperature is increased ...
Gay lussac's law worksheet.
State Charles and Gay-Lussac's Law in your own words: _____ 17. Blow up two balloons to the same size, about 6 inches in diameter. Place the opening of one balloon over the opening of each plastic bottle.
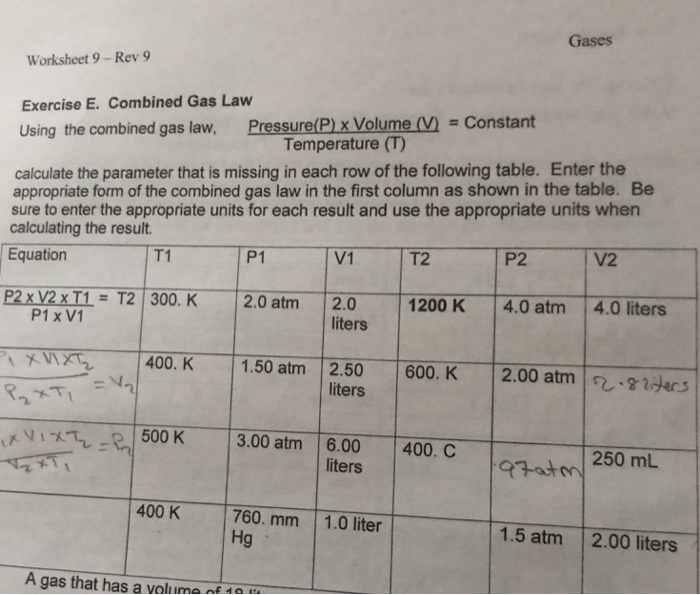
Combined Gas Law: Combines Boyle's, Charles', and Gay-Lussac's laws into one expression. With this equation we can see how changing more than one variable affects our unknown. P 1 V 1 /T 1 = P 2 V 2 /T 2. Ideal Gas Law: An ideal gas must follow the Kinetic Molecular Theory of Gases. We have talked about four variables that affect the ...
Joseph_Louis_Gay-Lussac) Joseph Louis Gay-Lussac (1778-1850), a French chemist and physicist, is known for his studies on the physical properties of gases. A balloon enthusiast as well, in 1804 he made a hot-air balloon ascent to a height of 20,000 feet in an early investigation of the Earth's atmosphere, and in 1805, discovered that the ...
Gay-Lussac's Law Worksheet: Annotate Problems on THIS sheet show work separately. 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 ˚C to 30.0 ˚C. 2. A container of gas is initially at 0.500 atm and 25 ˚C. What will the pressure be at 125 ˚C? 3.
The quiz and worksheet combination will check your understanding of Gay-Lussac's Law and how it relates to pressure and temperature. Quiz & Worksheet Goals During the assessments, you will be...
Gay-Lussac's Law Worksheet: 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 °C to 30.0 °C.
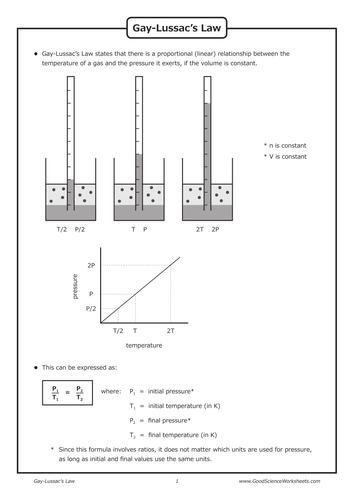
Gay-Lussac's law is a gas law which states that the pressure exerted by a gas (of a given mass and kept at a constant volume) varies directly with the absolute temperature of the gas. In other words, the pressure exerted by a gas is proportional to the temperature of the gas when the mass is fixed and the volume is constant.
The equation for Gay-Lussac's Law is: T1 = Initial Temperature ( Kelvin - K) P1 = Initial Pressure ( atm or mmHg) T2 = Final Temperature ( Kelvin - K) P2 = Final Pressure ( atm or mmHg) Note: Temperature must be in Kelvin for the equation to work. You calculate Kelvin temperature by adding 273 to the Celsius temperature.
This next example uses Boyle's Law as well as Gay-Lussac's Law. Twice the fun! Example #7: Two containers of identical volume are connected by a tube with a stopper to prevent gases inside the tanks from mixing with each other. Container A has a pressure of 2.00 atm and a volume of 10.0 L while container B has a pressure of 4.00 atm and a volume of 10.0 L.
4. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 °C to 30.0 °C. 1.03 atm (Gay-Lussac's Law) 5. If a gas is cooled from 323.0 K to 273.15 K and the volume is kept constant what final pressure would result if the original pressure was 750.0 atm? 634.2 atm (Gay-Lussac's Law) 6.
Unit 11 Packet - Page 4 of 14 Gay-Lussac's Law: P 1/T 1 = P 2/T 2 (P varies directly with Kelvin Temperature) Like which other law? You Try: 0Before a trip from Raleigh to NY, the pressure in a tire is 1.8 atm at 20 C. At the end of the trip the pressure gauge reads 1444 mm Hg.
Gay-Lussac's Law Worksheet. When volume is constant, 𝑃𝑃. 1. 𝑇𝑇. 1 = 𝑃𝑃. 2. 𝑇𝑇. 2. or P. 1. T. 2 = P. 2. T. 1. This gives you the Gay-Lussac's Law equation, where pressu re and temperature have a direct relationship. 1. What temperature units must all calculations for gas laws use:_____KELVIN_____. ...
Displaying top 8 worksheets found for - Lussac Gas Law Answer Keky. Some of the worksheets for this concept are Name gay lussac law work, Work 37 gas laws answers, Gas laws work 3 answers, Gas laws work 2 answers, Gas laws work 2 answers, Gas laws work 1 answers, Gas laws work 2 answers, Work 11.
Write the formula equation for Charles and Gay-Lussac's Law. V = CT. 11. Write the equation for Charles and Gay-Lussac's Law in words. For a given mass at constant pressure, volume is directly proportional to temperature. 12. In the animated gas lab, the unit of temperature is Kelvin. 13.
Guy-Lussac's Law 1. The gases in a hair spray can are at a temperature of 27 oC and a pressure of 30 lbs/in2. If the gases in the can reach a pressure of 90 lbs/in2, the can will explode. To what temperature must the gases be raised in order for the can to explode? Assume constant volume. (630 oC) 2.
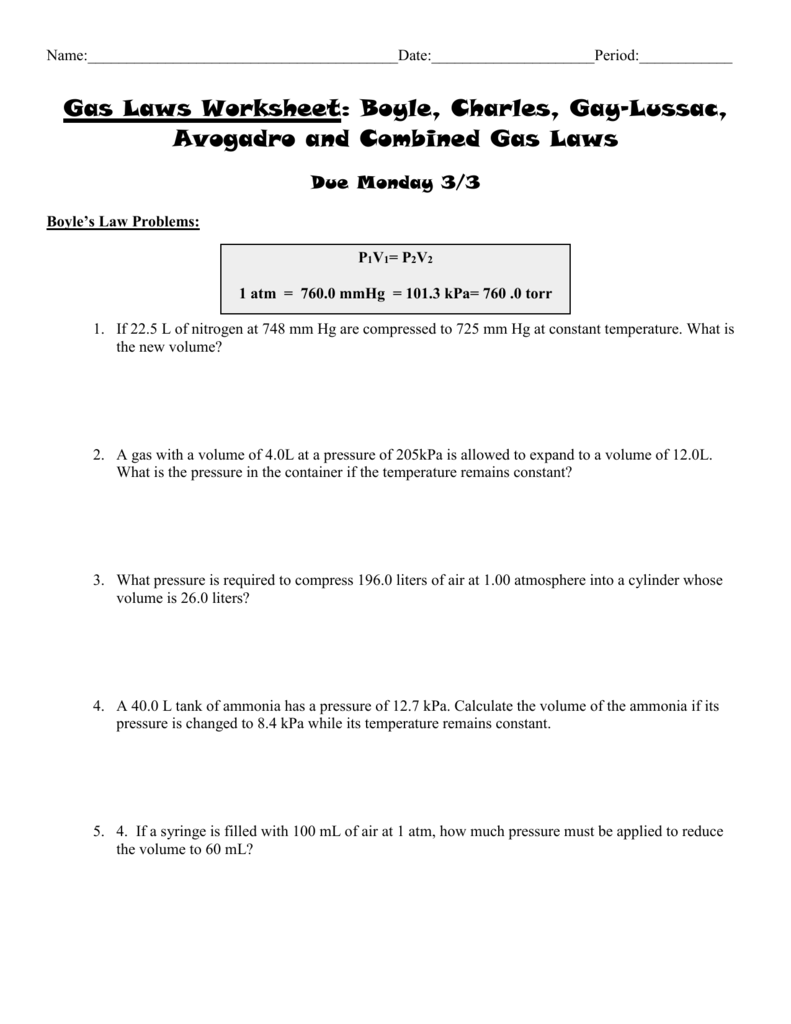
Gas Laws Worksheet #1 - Boyle's, Charles', Gay-Lussac's, and Combined Gas Law Boyle's Law: V 1 P 1 = V 2 P 2 1. A gas sample contained in a cylinder equipped with a moveable piston occupied 300.0 mL at a pressure of 2.00 atm. What would be the final pressure if the volume were increased to 500.0 mL at constant temperature? 2.
Gas Laws Worksheet #1 - Bo le's Charles' Ga -Lussac's and Combined Gas Law Solve all problems — you must show your work (including units). The correct answer is given in parentheses at the end of the problem. Boyle's Law 1. A as ple contained in a cylinder equipped with a moveable piston occupie 00.0 at a pressure
Gay-Lussac's Law Worksheet. 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 °C to 30.0 °C. 2. A gas has a pressure of 0.370 atm at 50.0 °C. What is the pressure at standard temperature? 3. A gas has a pressure of 699.0 mm Hg at 40.0 °C. What is the temperature at standard pressure?
This is Gay-Lussac's law. k=P/T. Explore. 20 minutes. Give students some an opportunity to practice working with the formula for Gay-Lussac's Law. k=P/T. Hand out the student worksheet and work together to solve the first few problems as a class before turing them loose tot work independently.
Name:_____ Gay-Lussac's Law Worksheet With Answers 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 ˚C to 30.0 ˚C. 1.03 atm 2. A container of gas is initially at 0.500 atm and 25 ˚C. What will the pressure be at 125 ˚C? 0.668 atm
This is a single 4-page worksheet covering the major empirical gas laws: Boyle's Law, Charles' Law, and Gay-Lussac's Law. There are conceptual and calculation-based questions for each law, and a "mixed review" that includes combined gas law problems. There are 12 problems total.Answer key is include. Subjects:
Gay-Lussac's Law Worksheet. A 3.00 L sample of nitrogen inside a rigid, metal container at 20.0 degrees Celsius is placed inside an oven whose temperature ...
Gay-Lussac's Law Worksheet With Answers 1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 ˚C to 30.0 ˚C. 2. A container of gas is initially at 0.500 atm and 25 ˚C. What will the pressure be at 125 ˚C? 3. A gas container is initially at 47 mm Hg and 77 K (liquid nitrogen temperature.)
Gay-Lussac's Law Worksheet . Assume that the volume and the amount of gas are constant in the following problems. 1. A gas in a sealed container has a pressure of 125 kPa at a temperature of 30.0°C. If the . pressure in the container is increased to 201 kPa, what is the new temperature? 2. The pressure in an automobile tire is 1.88 atm at 25 ...
Guy-Lussac's Law. The Law: P1 = P2 . T1 T2. Try This: Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0 ˚C to 30.0 ˚C.

Gas law practice problems: boyle's law, charles law, gay lussac's, combined gas law; crash chemistry






























0 Response to "43 gay lussac's law worksheet"
Post a Comment