41 chemistry worksheet heat and calorimetry problems
Calorimetry Quiz : ChemQuiz.net Calorimetry Quiz. This online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅ΔT) with options for different units of heat and temperature. Select your preferences below and click 'Start' to give it a try! Number of problems: 1. 5. 10. ICSE Books free Download PDF for Class 6 to 10 Verkko31. maalisk. 2021 · Class 9 Chemistry Concise Selina (Dr. S.P. Singh) Download Chapter wise ICSE Book pdf for Class 9 Chemistry Concise Selina (Dr. S.P. Singh) given below: ICSE Class 9 Chemistry Dr SP Singh Contents. ICSE Class 9 Chemistry Chapter 01 The Language of Chemistry. ICSE Class 9 Chemistry Chapter 02 Chemical Changes …
Heat Capacity Calorimetry Worksheet answers - StuDocu Heat Capacity/Calorimetry Worksheet Which kind of substance needs more energy to undergo an increase of 5 oC, something with a high or low Explain. High specific heat. cp are inversely proportional q/ cp ≈ ∆T , so if specific heat is high then energy will need to be high in order to maintain a similar temperature

Chemistry worksheet heat and calorimetry problems
College Chemistry Practice Tests - Varsity Tutors VerkkoThough integrating chemistry concepts into one’s knowledge base can seem difficult, there are dozens of college chemistry practice tests available from Varsity Tutors’ Learning Tools to assist you. The free college chemistry practice tests can help you brush up on your skills and identify any weaknesses you may have. 10.2 Calorimetry - Chemistry Fundamentals - University of Central Florida In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, M, to the cool water, W, until (b) both are at the same temperature. Example 10.2.1: Heat Transfer between Substances at Different Temperatures A 360-g piece of rebar (a steel rod used for reinforcing concrete) is dropped into 425 mL of water at 24.0 °C. Honors Chemistry Worksheet - Specific Heat Honors Chemistry Worksheet - Specific Heat Recognize that when two systems at different temperatures meet, there will be a net transfer of heat (energy) from the system of greater heat intensity to the system of lower heat intensity. Summary - Heat flows from source to sink, in other words from hot to cold until thermal equilibrium is obtained.
Chemistry worksheet heat and calorimetry problems. Worked Chemistry Problem Examples - ThoughtCo Verkko22. marrask. 2019 · This is an alphabetical list of worked example chemistry problems. Printable worksheets with questions and answers are also provided. Menu. Home. Science, Tech, ... Calorimetry & Heat Flow to D: Dilutions From Stock Conversions . Calorimetry & Heat Flow; ... Practicing Balancing Chemical Equations — Worksheet #2; Thermochemistry Worksheet + Answers - ChemistNate Step 1: Download this workbook which contains full solutions: . Thermochemistry-Worksheets-with-Full-Solutions-ChemistNate-July-2021.pdf. Download File. Step 2: Do the questions, and follow along with this video for when you get stuck: Study With Me: 90 Minutes of Thermo/Enthalpy/Heat Practice. Heat and Calorimetry Practice worksheet 1 (1).docx - Heat... Heat and Calorimetry worksheet Specific Heat Capacity, cH2O is 4.18 J/g ºC Please review Lesson 1 & 2, to complete this worksheet. 1. How many joules are needed to warm 25.5 grams of water from 14ºC to 22.5ºC? (ans. 9.1 x 102J) 2. Calculate the number of joules released when 75.0 grams of water are cooled from 100.0ºC to 27.5ºC. Free PDF Chemistry Worksheets To Download or Print - ThoughtCo Color Printable Periodic Table - Pretty much everything you need that can fit on a page and still be readable. Color table with atomic numbers, element symbols, element names, atomic weights, periods, and groups. [2013 Edition] [2012 Edition]Black/white Printable Periodic Table - Black/white table with atomic numbers, element symbols, element names, atomic weights, periods.
Quiz & Worksheet - Calorimetry | Study.com Calorimetry is a complicated science. This quiz/worksheet will help you assess your understanding of how to calculate temperature and heat capacity and let you put your skills to the test with ... This quiz and corresponding worksheet will gauge your … VerkkoQuiz & Worksheet... In chemistry, molarity (the moles of solute per liter of solution) is the solution concentration ... 2020 · Chemistry problems can vary in many different ways. Some questions are conceptual and ... September 2016, issue 9. The 70th Calorimetry Conference Special Issue. August 2016, issue 8. July 2016, issue 7.Jun 2019 ... Calorimetry Practice Problem Worksheets - Learny Kids Calorimetry Practice Problem Worksheets - total of 8 printable worksheets available for this concept. Worksheets are Calorimetry work w 337, Ii calori... Learny Kids; Home; Common Core. Math. Kindergarten; Grade 1; Grade 2; Grade 3; Grade 4; Grade 5; Grade 6; Grade 7; Grade 8; ELA ... Calorimetry Practice Problem - Printable Worksheets Some of the worksheets displayed are Calorimetry work w 337, Ii calorimetry work, Chemistry work heat and calorimetry problems answers, Calorimetry practice problems answers, Thermochemistry work 1, Calorimeter practice with answers, Calorimetry practice problems with answers, Calorimetry problems with answers.
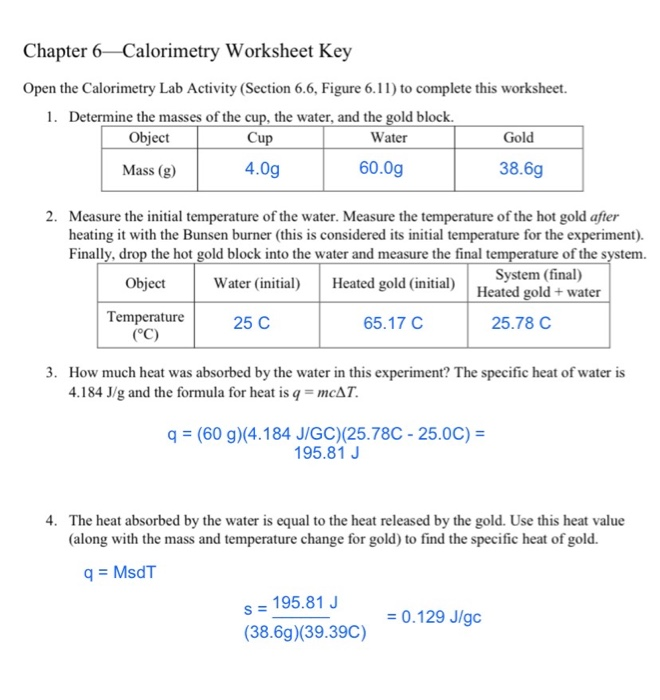
Calorimetry Problems 1 Worksheet Answer Key 8.2: Calorimetry (Problems) - Chemistry LibreTexts. Jan 29, 2021 ... Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g °C. ... Bookmark File PDF Chemistry Heating Curve Worksheet Answers ... Chemistry Heating Curve Answer Key Worksheets - Kiddy Math. Heating ... calculating specific heat worksheet Worksheet. 17 Images about Worksheet : Specific Heat Calculations Worksheet, Specific Heat Calculations Worksheet — db-excel.com and also Worksheet Calculations Involving Specific Heat — db-excel.com. Chemistry - Wikipedia VerkkoChemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the … PDF Calorimetry Practice Problems temperature, determined to be 27.8 °C. Assuming no heat lost to the environment, calculate the specific heat of the metal. (Hint: First calculate the heat absorbed by the water then use this value for "Q" to determine the specific heat of the metal in a second calculation) 6. In a coffee-cup calorimeter, 100.0 g of H 2
8.2: Calorimetry (Problems) - Chemistry LibreTexts The addition of 3.15 g of Ba (OH) 2 •8H 2 O to a solution of 1.52 g of NH 4 SCN in 100 g of water in a calorimeter caused the temperature to fall by 3.1 °C. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the following equation:
Join LiveJournal VerkkoPassword requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
How To Solve Basic Calorimetry Problems in Chemistry The Organic Chemistry Tutor 5.54M subscribers Join Subscribe This chemistry video tutorial explains how to solve basic calorimetry problems. It discusses how to calculate the heat energy...
PDF Calorimetry Worksheet - Laney College Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C)(55.4 °C) H = 1310 kJ
ICSE Board Books PDF Download - StudiesToday VerkkoChemistry (Dr. Viraf J Dalal) is written strictly in accordance with the latest syllabus prescribed by The Council for the Indian School Certificate Examination (CISCE). ICSE Board Books for Class 9 Chemistry include Basic Concept about each topic, Exercises, Lab Activity, Practice Worksheet, Value Based Questions, and Chapter-wise Summary.
PDF Calorimetry Worksheet W 337 - Everett Community College heat of combustion of the compound is 1,160 kJ/mol, what is the molar mass of the compound? 0.500 L H 2 O = 500 mL H 2 O = 5.00 x 10 2 g H 2 O H = (5.00 x 102 g H 2 O)(4.184 J/g 0C)(48.00 C) = 100000 J H = 100 kJ Now that we have the heat generated by the combustion of the compound we divide it by the given molar heat of combustion to find the ...
Thermochemistry questions (practice) | Khan Academy Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere. ... Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity.
Solving a Basic Calorimetry Problem | Chemistry | Study.com Step 1: Identify the mass of the substance and the specific heat capacity constant for the substance. Step 2: Identify the change in temperature by {eq}\triangle T = T_ {final} - T_ {initial}...
Calorimetry Practice Problem Worksheets - K12 Workbook Worksheets are Calorimetry work w 337, Ii calorimetry work, Chemistry work heat and calorimetry problems answers, Calorimetry practice problems answers, Thermochemistry work 1, Calorimeter practice with answers, Calorimetry practice problems with answers, Calorimetry problems with answers. *Click on Open button to open and print to worksheet. 1.
Algebrator online - softmath Verkkobinomial product worksheet ; Prentice Hall Algebra 2 with Trigonometry even answers ; adding and subtracting negative numbers worksheet ; quadratic formula calculator with complex roots ; math lesson plan instructions changing a decimal into a fraction ; sats yr6 questions for kids ; Aptitude questions +download ; trig equation solver
PDF Chemistry worksheet heat and calorimetry problems answers - JL Gardner check your answers. Heat and calorimetry problems of the chemical worksheet. Onora the specific heat chemistry worksheet recognizes that when two systems at different temperatures meet there will be a net transfer of thermal energy from the system of greater heat intensity to the system of less heat intensity. The heat of the reaction is. Look ...
Chemistry 101 - Introduction and Index of Topics - ThoughtCo Verkko2 päivää sitten · Chemistry is a science, but it is also used in human communication and interaction, cooking, medicine, engineering, and a host of other disciplines. Although people use chemistry every day with no apparent problem, if the time comes to take a course in chemistry in high school or college, many students are filled with dread.
CHEM 105 Problem Set 31 - Problem Set 31 - Calorimetry Chem ... - StuDocu CHEM 105 Problem Set 31 - Problem Set 31 - Calorimetry Chem 105 (a) What is calorimetry? - Studocu problem set 31 calorimetry chem 105 what is calorimetry? experimental method wherein changes in temperature are used to determine the energy released or DismissTry Ask an Expert Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew
Heat capacity and calorimetry (practice) | Khan Academy Heat capacity and calorimetry (practice) | Khan Academy Heat capacity and calorimetry AP.Chem: ENE‑2 (EU), ENE‑2.D (LO), ENE‑2.D.1 (EK), ENE‑2.D.2 (EK), ENE‑2.D.3 (EK), ENE‑2.D.4 (EK), ENE‑2.D.5 (EK), ENE‑2.D.6 (EK) AP® is a registered trademark of the College Board, which has not reviewed this resource.
Chemistry Quizzes | Study.com VerkkoInterested in seeing how well you know a particular chemistry concept? Take Study.com's brief multiple-choice quiz. Obtain rapid feedback and results to understand how well you performed. The quiz ...
Calorimetry Problems Answer Key PDF Calorimetry Problems Worksheet Answer Key If the heat capacity of the calorimeter is 21.6 kJ/°C, determine the heat produced by combustion of a ton of coal (2000 pounds). Remember 1 kg = 2.2 pounds Answer 2.91 x 107 kJ PROBLEM \ (\PageIndex {10}\) A teaspoon of the carbohydrate sucrose (common sugar) contains 16 Calories (16 kcal).
Calorimetry Practice Problem Worksheets - Lesson Worksheets Displaying all worksheets related to - Calorimetry Practice Problem. Worksheets are Calorimetry work w 337, Ii calorimetry work, Chemistry work heat and calorimetry problems answers, Calorimetry practice problems answers, Thermochemistry work 1, Calorimeter practice with answers, Calorimetry practice problems with answers, Calorimetry problems with answers.
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity ... This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. It shows you how to calculate the quantity of heat transferred using specific heat capacity...
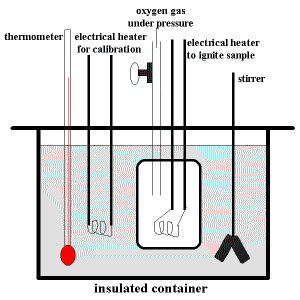
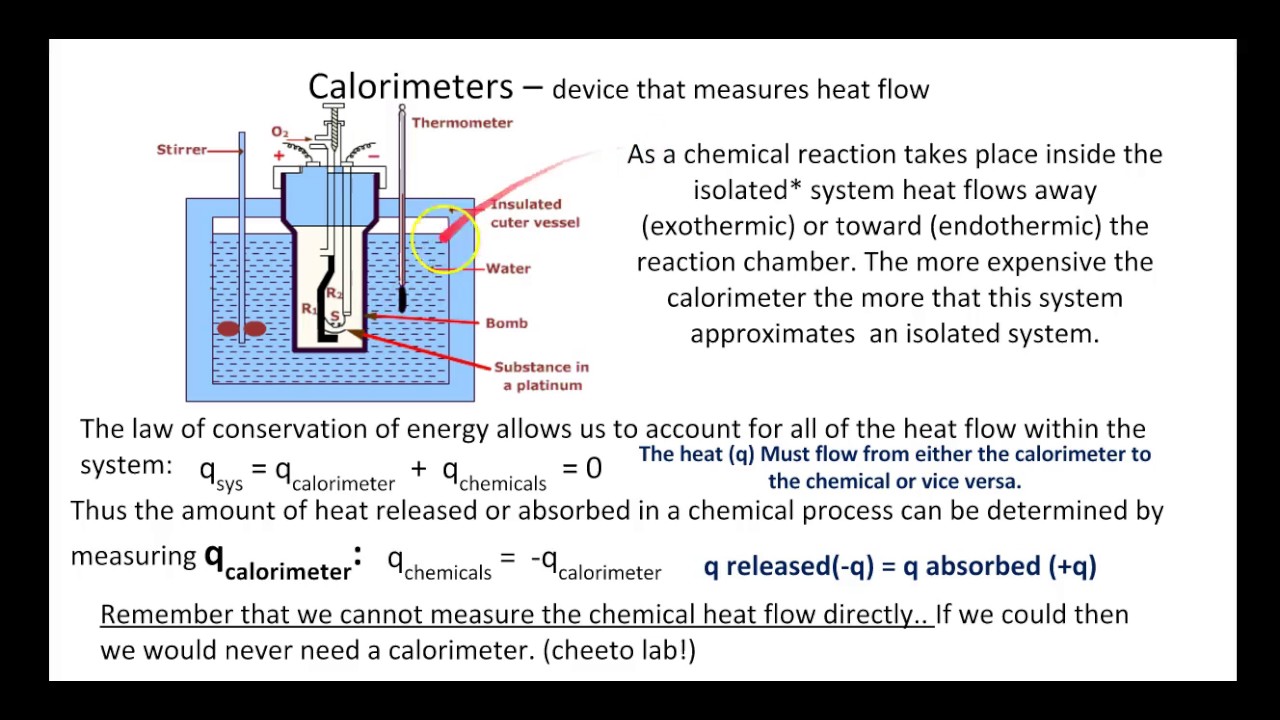
Calorimetry and Heat Flow: Worked Chemistry Problems - ThoughtCo Calorimetry is the study of heat transfer and changes of state resulting from chemical reactions, phase transitions, or physical changes. The tool used to measure heat change is the calorimeter. Two popular types of calorimeters are the coffee cup calorimeter and bomb calorimeter.
8.5.1: Practice Problems- Calorimetry - Chemistry LibreTexts The addition of 3.15 g of Ba (OH) 2 •8H 2 O to a solution of 1.52 g of NH 4 SCN in 100 g of water in a calorimeter caused the temperature to fall by 3.1 °C. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the following equation:
Honors Chemistry Worksheet - Specific Heat Honors Chemistry Worksheet - Specific Heat Recognize that when two systems at different temperatures meet, there will be a net transfer of heat (energy) from the system of greater heat intensity to the system of lower heat intensity. Summary - Heat flows from source to sink, in other words from hot to cold until thermal equilibrium is obtained.
10.2 Calorimetry - Chemistry Fundamentals - University of Central Florida In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, M, to the cool water, W, until (b) both are at the same temperature. Example 10.2.1: Heat Transfer between Substances at Different Temperatures A 360-g piece of rebar (a steel rod used for reinforcing concrete) is dropped into 425 mL of water at 24.0 °C.
College Chemistry Practice Tests - Varsity Tutors VerkkoThough integrating chemistry concepts into one’s knowledge base can seem difficult, there are dozens of college chemistry practice tests available from Varsity Tutors’ Learning Tools to assist you. The free college chemistry practice tests can help you brush up on your skills and identify any weaknesses you may have.

























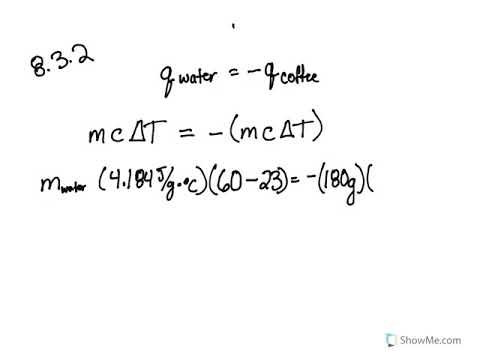


0 Response to "41 chemistry worksheet heat and calorimetry problems"
Post a Comment